Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
Benzil (BZ) can be converted almost quantitatively to benzoyl peroxide (BP) in aerated polymer films upon irradiation at >400 nm (i.e., the long-wavelength edge of the n→π* absorption band of BZ, where BP does not absorb).
- benzil
- photoperoxidation
- benzoyl peroxide
- polymeric benzoyl peroxide
- crosslinking
- polystyrene
1. Introduction
Photon-induced transformations of 1,2-diphenylethanedione (benzil, BZ) and related compounds into benzoyl peroxide (BP) in glassy polymer matrices are the basis for this review. It will primarily treat photoperoxidations induced by molecular oxygen of BZ, compounds containing two BZ structures dissolved in polymers, and BZ groups covalently appended to polymers. The courses of the subsequent thermal or photochemical decompositions of the resultant BP photoproducts and their influence on the polymer properties are also described. They lead to a variety of crosslinks in the polymers. Polystyrene (PS) is featured here as a polymer for investigation.
A common method for synthesizing crosslinked PS involves copolymerization of styrene and a monomer with two or more vinyl groups. However, this method is not readily feasible for the preparation of crosslinked thin polystyrene films due to chemical, environmental and other considerations that accompany polymerization. Such films are prepared usually by the removal of solvents from solutions on substrates. Although less frequently used, post-polymerization crosslinking of PS offers much greater flexibility and specificity, especially in those cases which require radiation to form the needed crosslinks (e.g., the transformation of polymeric materials, such as photoresists, into micron- and submicron-sized structures for microelectronics [1][2]). Currently, the most commonly employed photoresists involve reactions that create photo-acids or photo-bases [3] in situ [4][5].
Selective surface modification and functionalization processes that involve the introduction of crosslinks have become increasingly important today to produce chemical or biosensors, especially for classical resist applications in the fields of interface engineering and nanotechnology [6][7]. Photo- or thermo-active agents to achieve these crosslinks have included a variety of reagents (e.g., nitrenes, carbenes, excited triplet states of carbonyl-containing compounds or sensitized decomposition of a perester) that can be covalently linked to a polymer or added as a low-molecular dopant containing two active groups that can react directly with a polymeric substrate to form a network [1]. In this regard, the photoperoxidation of BZ structures in the air (by molecular oxygen) is well suited to produce crosslinks within thin layers of polymers.
Additionally, there are clear advantages to linking the crosslinking agent directly to a polymer over adding a non-covalently linked dopant. Principal among these are the higher efficiency of crosslinking and the inability of the active units to separate into microphases over time, thereby reducing the efficiency of crosslinking and the distribution of the crosslinks; well-defined layers, patterns and images may be obtained. However, in all cases, the crosslinking agent must be compatible with and appropriate for the type of polymer being employed.
There are many specific examples of how these issues have been addressed. For instance, self-crosslinkable polymers have been prepared by post-modification of PS with reactive groups such as maleinimide [8] or iodide [9] or by post-modification of poly(4-chloromethyl styrene) with light-sensitive structures such as benzophenone, stilbazolium, fluorenone, carbazole and sulphonyl azide [10]. Self-crosslinkable PS can also be prepared by copolymerization of styrene and a monomer containing a reactive functional group (e.g., azide [11] and phenylindene [12]). The photosensitized cleavage of peresters in copolymers containing p-vinylbenzophenone-p’-tert-butyl perbenzoate units has been found to be reasonably efficient [13][14]. Additionally, high crosslinking efficiency in surface layers of nanometer-range thicknesses has been induced by the thermal coupling reaction of two pendant benzocyclobutene structures [15] in melts and by azide-alkyne [16] groups.
Low-molecular-weight crosslinking agents such as very reactive difunctional Friedel-Crafts reagents [17] have been used in bulk with PS, and bisazides [18] have been doped into synthetic polymers for crosslinking their films. Perfluorophenylazides, which produce very high insertion yields, have been used to introduce functional groups, especially into synthetic polymers [7]. Additionally, a highly efficient negative photoresist based on poly(vinyl phenol) has been developed using bis(perfluorophenyl azides) as the crosslinking agent [19]. A major advantage of low-molecular-weight reagents is the possibility to use one dopant for crosslinking a variety of polymers. Photoperoxidation and crosslinking by a low-molecular-weight molecule containing two 1,2-dicarbonyl groups, doped into PS films, has been shown to be a very efficient crosslinking method (vide infra). BZ-containing dopants are more soluble than the corresponding BP-containing dopants in many polymers. As a result, the BZ reagents can usually effect higher concentrations and more even distributions of crosslinks than can the BP ones. Moreover, the more thermally stable BZ groups can be heated, shaped, extruded, blended, etc. in polymer processing steps prior to the thermal initiation of crosslinking in which the thermally sensitive BP initiators are generated in situ.
Diacylperoxides and many dialkyazo compounds are exploited frequently in polymer chemistry as polymerization initiators and crosslinkers due to their ease of thermal decomposition to form radicals. Although there is ample literature on polymeric azo radical initiators, [20] the formation and subsequent use of polymeric diacylperoxides has not been reported previously and is summarized here.
2. Examples of the Application of Benzil Photoperoxidations
The feasibility of employing VBZ/S as a negative photoresist (i.e., to form non-erasable images) has been demonstrated [21]. A spin-coated 0.5 μm thick film on a silicon wafer was irradiated through a mask at 254 nm. The irradiated part became highly crosslinked, and the unirradiated parts could be dissolved in isobutyl methyl ketone to develop the image. The pattern after irradiation contained features comparable in size to the smallest apertures of the mask.
The advantages of using pre-crosslinked positive resists have been discussed [22]. Irradiation (>400 nm) in the air of a copolymer of phenyl vinyl ketone (PVK) with VBZ (PVK/VBZ) containing 1.5 wt% of VBZ structural units [23] in the film (for the photoperoxidation step), followed by thermolysis of the resulting pendant BP groups, leads to crosslinking. Subsequent irradiation at 366 nm of the crosslinked polymer causes cleavage of the PVK chain between junction points of the polymer network through Norrish type II reactions (Scheme 1) [24][25][26][27]. Thus, the PVK/VBZ copolymer represents a novel type of photoresist based on polymer network de-crosslinking. The process involves three steps: (1) photo-generation of peroxide-containing moieties; (2) crosslinking via thermolysis of the peroxides; and (3) subsequent photoinduced de-crosslinking of the polymer network. This material provides positive-tone images after UV exposure (>330 nm) and development in an organic medium, such as isopropyl methyl ketone.
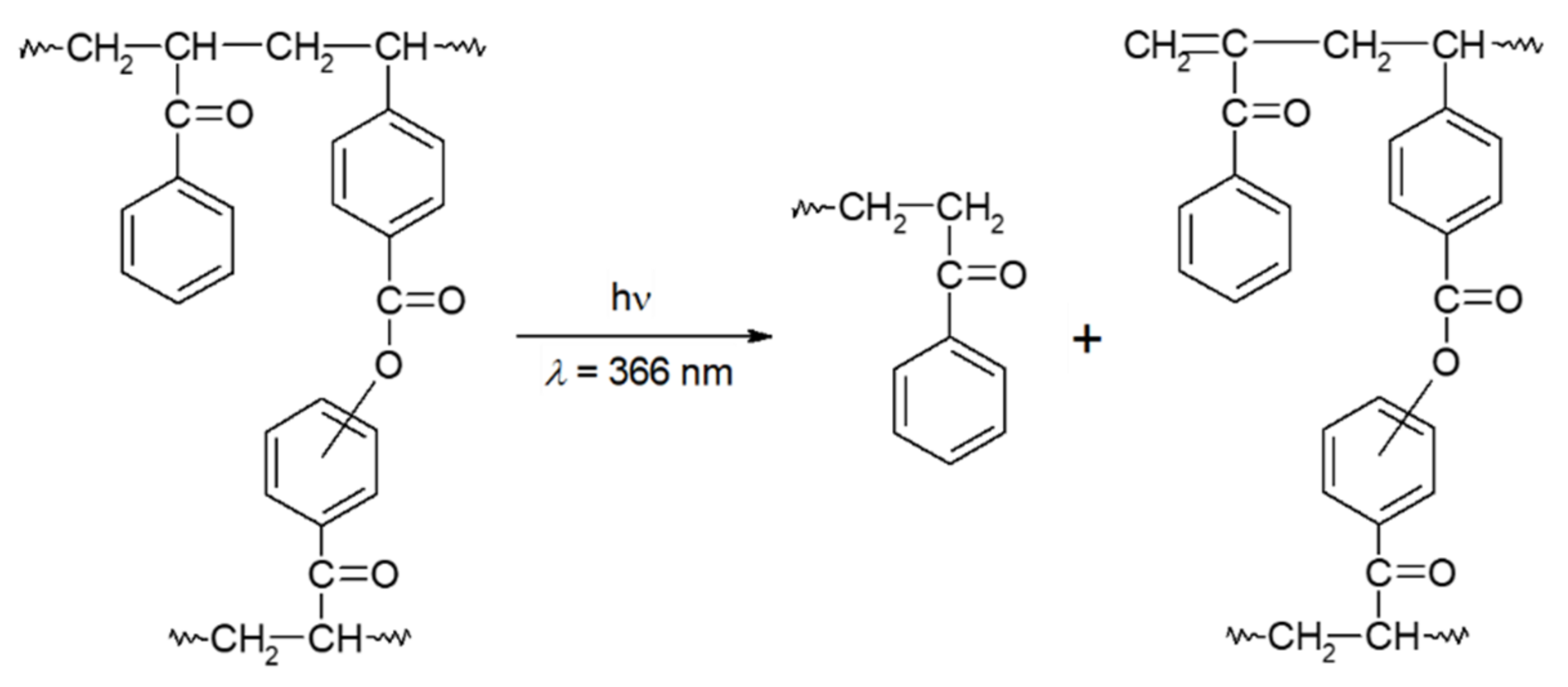
Scheme 1. Cleavage of PS chains via Norrish type II photoreactions.
Decomposition of photogenerated BP groups can be utilized for surface modification by grafting [28]. Thermal decomposition of BP groups of a photoperoxidized BZMA/S was successfully utilized for grafting by immersing the film in a boiling 15% aqueous solution of methacrylic acid. Additionally, photosensitized decomposition (λ = 366 nm) of BP groups of a photoperoxidized PCOCO/S film was used as a graft by immersing it in acrylic acid. Some examples of applications of the photoperoxidation of pendant copolymer BZ groups are presented in Table 2.
Table 2. Some examples of applications of photoperoxidation of pendant copolymer BP groups.
| Copolymer | Application | [Ref.] |
|---|---|---|
| VBZ/S | Negative photoresist (254 nm) | [21] |
| PVK/VBZ | Positive photoresist based on polymer network de-crosslinking | [23] |
| BZMA/S | Thermal grafting of methacrylic acid | [28] |
| PCOCO/S | Photografting (366 nm) of methacrylic acid | [28] |
| BZMA/MMA | Copolymer grafting on polyethylene surfaces | [28] |
3. Conclusions
The photochemistry of BZ in solution leads to various products whose nature depends on the presence or absence of molecular oxygen, hydrogen-atom donors, the wavelength of irradiation, temperature and reactant concentration.
Upon irradiation at >370 nm, BZ, either doped or covalently bound to a polymer matrix, can be converted to BP almost quantitatively. The extent of conversion depends on the nature of the substituents on the BZ structure. The presence of side products can be detected conveniently by FTIR spectroscopy in frequency regions between where the diagnostic peaks of the starting BZ and the BP peroxide products appear.
Cleavage of photochemically generated BP groups bound to a polymer backbone leads to extensive crosslinking by the addition of intermediate benzoyloxy macroradicals to aromatic rings of PS or other aromatic polymers.
The proposed mechanism of the PS crosslinking in the thermal decomposition of compounds containing two BP groups (BP-O-BP) is based on correlations of crosslink density (concentration of crosslinks, concentration of junction points) and concentrations of BP-O-BP (at low concentrations of BP-O-BP). From these correlations, one crosslink requires four BP-O-BP structures. The yield of acyloxy radicals from thermal scission of BP-O-BP is quantitative. Each acyloxy radical can add to an aromatic ring or accept a hydrogen atom from a benzoyloxy radical–phenyl ring addition complex with equal probability. For the peroxides generated from compounds containing two BZ structures (BZ-O-BZ) in a polymer film, the crosslink density depends on the yield of the photooxidized product containing two BP groups (N. B., BP-O-BP).
Because of the high reactivity of benzoyloxy radicals, the approaches described here should be amenable to crosslinking a wide variety of other polymers besides PS. Crosslinkers with two covalently attached BZ groups offer two important practical advantages over those containing two covalently attached BP structures: (1) they are safer to handle; (2) they are usually more soluble in polymers.
However, crosslinking with a BZ-type crosslinker can be achieved in two steps by irradiating initially (at >370 nm) in the presence of molecular oxygen followed by thermal cleavage of the as-produced BP compounds to generate benzoyloxy radicals. Alternatively, crosslinking can occur in one step by irradiation at shorter wavelengths, where BP groups are formed and decomposed sequentially.
Self-supported PS films of thickness in the nm range cannot be prepared for reasons dealing with the mechanical properties of the polymer. Nano-thickness layers of PS film surfaces can be modified by crosslinking using BZ structures bound to styrene copolymer chains and using doping agents containing two BZ groups. Crosslinking by BP groups formed photochemically by irradiation that does not overlap with the BP absorptions can be initiated thermally. BP groups prepared from BZ groups in a VBZ/S copolymer irradiated at 254 nm are simultaneously decomposed with the same light, crosslinking only layers within ~2.4 µm of the surface due to strong absorption of the PS phenyl rings [21]. This depth is significantly greater than the nm range.
Clearly, the methods of crosslinking described here offer advantages over other published methods for the modification of surfaces and polymer films. Those advantages are described by several examples within this text.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175154
References
- Reiser, A. Photoreactive Polymers. The Science and Technology of Resists; John Wiley and Sons: New York, NY, USA, 1989.
- Turner, S.R.; Daly, R.C. The Chemistry of Photoresists. In Photopolymerisation and Photoimaging Science and Technology; Allen, N.S., Ed.; Elsevier: London, UK, 1989; pp. 75–113.
- Ito, H. Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: London, UK, 1993; p. 237.
- Miyagawa, K.; Naruse, K.; Ohnishi, S.; Yamaguchi, K.; Seko, K.; Numa, N.; Iwasawa, I. Study on thermal crosslinking reaction of o-naphthoquinone diazides and application to electrodeposition positive photoresist. Progr. Org. Coatings 2001, 42, 20–28.
- Suyama, K.; Tsunooka, M.; Shirai, M. Photoacids and photobase generation in photoresists. In Trends in Photochemistry & Photobiology; Fouassier, J.P., Ed.; Research Trends: Poojapura, India, 1999; Volume 5, pp. 169–185.
- Kern, W. Trends in Photochemistry & Photobiology; Fouassier, J.P., Ed.; Research Trends: Poojapura, India, 2001; p. 11.
- Pei, Y.; Yu, H.; Pei, Z.; Theurer, M.; Ammer, C.; André, S.; Gabius, H.-J.; Yan, M.; Ramström, O. Photoderivatized Polymer Thin Films at Quartz Crystal Microbalance Surfaces: Sensors for Carbohydrate–Protein Interactions. Anal. Chem. 2007, 79, 6897–6902.
- Stevens, M.P.; Jenkins, A.D. Crosslinking of polystyrene via pendant maleimide groups. J. Polym. Sci. Pol. Chem. 1979, 17, 3675.
- Ueno, T.; Shiraishi, H.; Nonogaki, S. Insolubilization mechanism and lithographic characteristics of a negative electron beam resist iodinated polystyrene. J. Appl. Polym. Sci. 1984, 29, 223–235.
- Gibson, H.W.; Bailey, F.C.; Chu, J.Y. Chemical modification of polymers-11. photoreactive polymers from poly(vinylbenzyl chloride). J. Polym. Sci. Pol. Chem. 1979, 17, 777–782.
- Morita, H. Photocrosslinking reaction of azidomethylated polystyrene in solid polymer matrices. J. Photopolym. Sci. Technol. 1991, 4, 225–230.
- Schinner, R.; Wolff, T.; Kuckling, D. Defined photocrosslinking and viscometric data I: Crosslinking via phenylindene and stilbazolium chromophores. Ber. Bunsenges. Phys. Chem. 1998, 102, 1710–1714.
- Gupta, I.; Gupta, S.N.; Neckers, D.C. Photocrosslinking and photografting of vinyl polymers using poly(styrene-co-p-vinylbenzophenone-p′-tert-butyl perbenzoate) as a comonomer. J. Polym. Sci. Pol. Chem. 1982, 20, 147–157.
- Neckers, D.C.J. Thirty-Five Years in Rad Cure:Taking Radiation Curing From Academia to Industry. Radiat. Curing 1983, 10, 19–26.
- Ryu, D.Y.; Shin, K.; Drockenmuller, E.; Hawker, C.J.; Russell, T.P. A Generalized Approach to the Modification of Solid Surfaces. Science 2005, 308, 236–239.
- Spruell, J.M.; Wolffs, M.; Leibfarth, F.A.; Stahl, B.C.; Heo, J.; Connal, L.A.; Hu, J.; Hawker, C.J. Reactive, Multifunctional Polymer Films through Thermal Cross-linking of Orthogonal Click Groups. J. Am. Chem. Soc. 2011, 133, 16698–16706.
- Grassie, N.; Gilks, J. Friedel-Crafts crosslinking of polystyrene. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 1531–1552.
- Yan, M. Photochemically Initiated Single Polymer Immobilization. Chem. Eur. J. 2007, 13, 4138–4144.
- Yan, M.; Wybourne, M.N.; Keana, J.F.W. Bis(perfluorophenyl azides) as highly efficient crosslinking agents for poly(vinyl phenol). React. Funct. Polym. 2000, 43, 221–225.
- Nuyken, O.; Voit, B. Polymer Frontiers International. In Macromolecular Design: Concept and Practice; Mishra, M.K., Ed.; Polymer Frontiers Intl: New York, NY, USA, 1994; p. 313.
- Mosnáček, J.; Weiss, R.G.; Lukáč, I. Preparation of 4-Vinylbenzil and Photochemical Properties of Its Homopolymer and Copolymer with Styrene. Macromolecules 2004, 37, 1304–1311.
- Li, M.Y.; Liang, R.C.; Reiser, A. Photodecoupling of Cross-Links in Polymeric Gels. Macromolecules 1990, 23, 2704–2709.
- Mosnáček, J.; Lukáč, I.; Chromik, Š.; Kostič, I.; Hrdlovič, P. Network Formation of a Phenyl Vinyl Ketone Copolymer with 4-Vinylbenzil and Its Photodecrosslinking in Films. J. Polym. Sci. Pol. Chem. 2004, 42, 765–771.
- Lukáč, I.; Hrdlovič, P.; Maňásek, Z.; Belluš, D. Influence of Free and Copolymerized Triplet Quenchers on the Photolysis of Poly(vinyl Phenyl Ketone) in Solution. J. Polym. Sci. A-1 1971, 9, 69–80.
- Hrdlovič, P.; Lukáč, I. Photolysis of poly(1-(4-substituted phenyl)-2-propen-1-ones: The Norrish Type II reaction in polymers. In Developments in Polymer Degradation; Grassie, N., Ed.; Applied Science Publishers: London, UK, 1982; Volume 4, pp. 101–141.
- Hrdlovič, P.; Lukáč, I. Light Degradation of Ketone Polymers. In Proceedings of the International Conference on Advances in Stabilization and Controlled Degradation of Polymers, Lucerne, Switzerland, 24–26 May 1989; pp. 66–78.
- Hrdlovič, P.; Scaiano, J.C.; Lukáč, I.; Guillet, J. Transient Spectroscopy and Kinetics of Poly(1-substituted-phenyl)-2-propen-1-ones). Macromolecules 1986, 19, 1637–1643.
- Mosnáček, J.; Lukáč, I.; Bertoldo, M.; Ciardelli, F. Applicability of photochemically generated pendant benzoyl peroxides in both “grafting from” and “grafting to” techniques. Chem. Pap. 2013, 67, 9–17.
This entry is offline, you can click here to edit this entry!
