Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Prostate cancer (PCa) is the leading cause of cancer-associated mortality in men, and new biomarkers are still needed.
- syndecan
- prostate cancer
- outcome
- prognostic marker
1. Introduction
Prostate cancer (PCa) is the leading cause of cancer-associated mortality in men worldwide [1]. The incidence of PCa in the global population may reflect increased life expectancy, improvements in the health information system, and screening practices using the prostate-specific antigen (PSA) test [2]. Despite advances in the detection of PCa, the main challenge is the difficulty in the early distinguishing of aggressive tumors from indolent ones in patients with a low-grade Gleason score [3]. Promising diagnostic and patient risk stratification approaches to prostate tumor cells have emerged, such as liquid biopsies and exosomes [4][5]. However, new biomarkers are still needed to improve both earlier diagnosis of PCa and patient stratification risk.
The syndecan (SDC) family consists of four transmembrane type I proteoglycans, syndecan-1, -2, -3, and -4 (SDC1–4), which are encoded by four different genes [6][7]. SDCs are differentially expressed in various tissues. SDC1 is found predominantly on the basolateral surface of epithelial cells [8][9]. SDC2 is mainly present in cells of mesenchymal origin, fibroblasts, and endothelial cells. SDC3 is expressed primarily by neuronal tissue and cartilage [10], and SDC4 is found in most tissues but has a relatively low abundance [11][12][13]. SDCs have been associated with cellular signaling, cell adhesion, migration, and exosome release [6][14][15][16].
Syndecans have a six-domain organization. The extracellular amino terminal is modified with glycosaminoglycan chains. All four family members have heparan sulfate changes. SDC1 and SDC3 have additional chondroitin sulfate chains. Proximal to the plasma membrane is a protease-sensitive cleavage site responsible for the shedding of the amino terminal glycosaminoglycan-bearing domain [16][17]. Following the transmembrane domain are three cytosolic carboxy-terminal domains. The highly conserved C1 and C2 domains are intercalated by a variable region. Multiple interactions with both the extracellular matrix and soluble cytokines and chemokines are mediated by the extracellular domain. The transmembrane domain is involved in homodimerization. The cytosolic conserved C1 domain is involved in interacting with the actin filaments via binding to ezrin, radixin, and moesin proteins. In its turn, the C2 domain interacts with synectin, syntenin, and calcium-calmodulin-associated serine/threonine kinase [16][18][19]. Readers are referred to references [19][20] for detailed reviews on syndecans.
The syndecans play an important role in the progression and prognosis of many types of cancer [21][22][23]. SDC1 is the best-characterized member of the SDC family and is expressed in all types of epithelial cells [18][24][25]. Loss of SDC1 is associated with tumor progression and poor prognosis in a variety of cancers [26][27][28]. However, observations described by Palaiologou et al. (2014) showed contradictory results in prostate, breast, ovarian, liver, and pancreatic cancer because SDC1 immunoexpression may change depending on tumor stage [29]. Additionally, syntenin-1 (syndecan binding protein, SDCBP or MDA9), which is important for syndecan signaling, has also been involved in cancer progression. SDCBP shows increasing expression in tumor progression from localized to metastatic lesions [30][31]. As mentioned above, SDCBP binds to the conserved syndecan C2 via its PDZ domains [31]. There is no information on the affinity of SDCBP to the different syndecans.
2. Sdc Family Members and Sdcbp mRNA Levels in GEMM of PCa
Reads per kilobase of transcript per million mapped reads (RPKM) for each lobe were grouped in a heatmap by condition and stage of progression to facilitate data visualization. Gene expression analysis from RNA sequencing data of two GEMM of PCa showed several essential changes in Sdc1–4 in prostate tumors at different stages of tumor progression. The level of Sdcbp expression did not differ significantly between stages of progression (Figure 1).
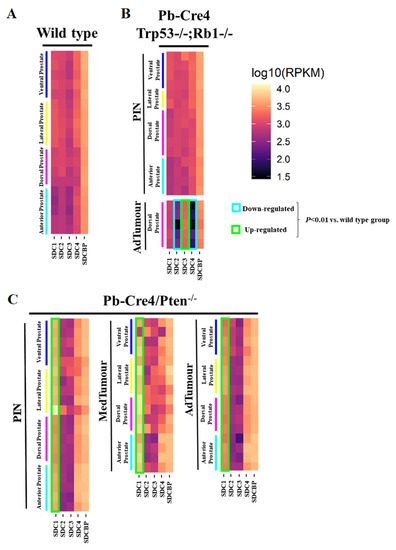
Figure 1. Heatmaps illustrate RNA-Seq differential expression data of the Sdc1, Sdc2, Sdc3, Sdc4, and Sdcbp genes across different prostatic lobes, mouse models, and tumor stages. (A) Non-neoplastic tissue (Pb-Cre4-negative—wild type) controls; (B) Pb-Cre4/Trp53f/f;Rb1f/f double conditional knockout mouse (p53/Rb mouse); (C) Pb-Cre4/Ptenf/f mouse (Pten mouse). The heatmaps represent Log10 of normalized RPKM values. PIN Stage—prostatic intraepithelial neoplasia; MedTumor—medium-stage tumor, micro-invasive adenocarcinomas; AdTumor—tumor in a more advanced stage, invasive adenocarcinomas. Note that significant upregulation of Sdc1 in the Pten mouse (*** p < 0.0001 vs. control group), a significant upregulation of Sdc3, and downregulation of both Sdc2 and Sdc4 in advanced tumors from the p53/Rb mouse were also observed (p < 0.0001 vs. wildtype group).
In non-tumor tissues, Sdc1, Sdc2, Sdc3, Sdc4, and Sdcbp were expressed in all four prostate lobes, with no significant quantitative difference among them. Only slightly lower expression of all five genes was observed in the AP than in the other three lobes (DP, LP, and VP) (Figure 1A).
No significant alteration was observed in the p53/Rb mouse. However, a substantial reduction in expression of Sdc2 was shown in advanced tumor stages in the p53/Rb mouse. No alteration was observed in the expression of the Sdc3 gene in the Pten mouse, although in the p53/Rb mouse, Sdc3 overexpression was observed. In the p53/Rb mouse, in advanced tumor stages, Sdc4 expression was significantly reduced. No significant alteration was observed in the Sdcbp gene expression pattern (Figure 1B).
In the Pten mouse, the number of RPKM for Sdc1 was compatible with increased expression across stages of tumor progression, in PIN lesions, and at medium and advanced stages (Figure 1C).
3. SDC Family and SDCBP Protein Expression in Knockout Mice Prostatic Tissues
Immunohistochemistry for SDC1-4 and its cytoplasmic anchoring protein, SDCBP, were performed on samples from different tumors. The non-tumoral prostatic tissue showed slight epithelial staining and intense staining around smooth muscle cells (Figure 2A). In the Pten mouse, strong immunostaining of SDC1 in the glandular epithelium in the PIN stage (Figure 2B) and in advanced undifferentiated tumors (Figure 2C) was observed. In the p53/Rb mouse, SDC1 was not observed in prostate stromal immunostaining in the PIN stage (Figure 3A) or advanced tumor stage (Figure 3B).
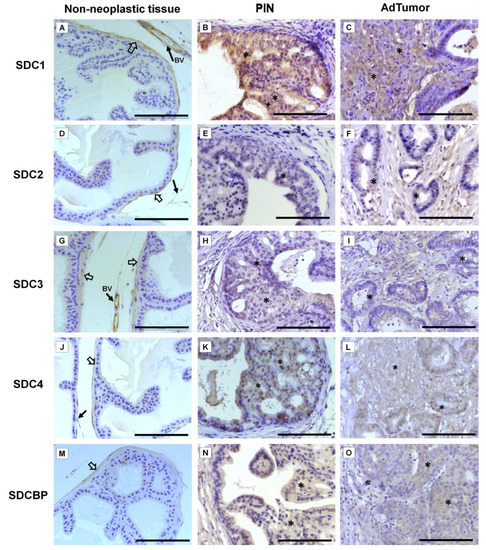
Figure 2. Representative images of the immunohistochemical staining for SDC1, -2, -3, and -4, and SDCBP in non-neoplastic tissues (Pb-Cre4-negative—wild type) and tumoral prostatic lobes from Pb-Cre4/Ptenf/f genetic engineered mouse model. (A–C) SDC1. (D–F) SDC2. (G–I) SDC3. (J–L) SDC4. (M–O) SDCBP. Prostatic intraepithelial neoplasia (PIN). Advanced tumors (AdTumor). In the non-neoplastic prostatic lobes, positive immunostaining was observed in the interstitial connective tissue (solid arrows), blood vessels (BV), and smooth muscle cells (open arrows). A weak or negative reaction was observed in the secretory epithelial cells for all SDCs and SDCBP. At the PIN and advanced stages of the tumor (asterisks), strong immunostaining for SDC1 in the tumoral epithelial cells, weak immunostaining for SDC4 and SDCBP, and negative immunostaining for SDC2 and SDC3 were observed. Scale bars: 100 μm.
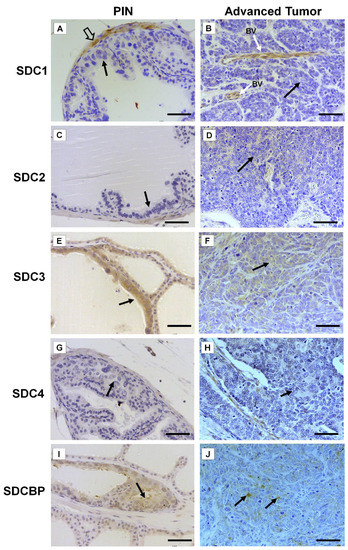
Figure 3. Representative images of the immunohistochemical staining for SDC1, -2, -3, and -4, and SDCBP in tumors found in the different prostatic lobes from the Pb-Cre4/Trp53f/f-;Rb1f/f genetically engineered mouse model. (A,B) SDC1; (C,D) SDC2; (E,F) SDC3; (G,H) SDC4; (I,J) SDCBP. Prostatic intraepithelial neoplasia (PIN) and advanced tumors (AdTumor). In the PIN areas and advanced tumors, positive immunostaining was observed for SDC3 (E) and SDCBP (I) (solid arrows). Blood vessels (BV); smooth muscle cells (open arrow). Scale bars: 100 μm.
SDC2 showed slight immunostaining in both Pten and p53/Rb mice for both the glandular epithelium and stroma in the PIN and advanced tumor stages (Figure 2D–F; Figure 2C,D). In the normal prostate (non-neoplastic tissue), SDC3 showed strong stromal immunostaining with a marked concentration around blood vessels (Figure 2G). SDC3 showed no reaction in the glandular epithelium of the PIN stage in the Pten mouse (Figure 2H), but SDC3 was poorly expressed in well-differentiated advanced tumors (Figure 2I). In the p53/Rb mouse, SDC3 immunostaining was predominant and intense in the glandular epithelium of PIN lesions (Figure 3E) and moderate in neoplastic cells of advanced tumors (Figure 3F).
SDC4 showed no immunostaining in the stroma of normal tissue (Figure 2J). SDC4 showed moderate epithelial immunostaining in the PIN stage (Figure 2K) from the Pten mouse. Epithelial and stromal immunostaining of SDC4 was present in the advanced stage in the Pten mouse (Figure 2L). There was no immunostaining in the PIN or advanced tumor stage for SDC4 in the p53/Rb mouse (Figure 3G,H). SDCBP showed poor immunostaining in the normal prostatic epithelium (Figure 2M). However, SDCBP immunostaining was moderate in tumor cells in the PIN and advanced stages of the tumor in the Pten mouse (Figure 2N,O). In the p53/Rb mouse, strong expression of SDCBP was observed only in the PIN stage, and no reaction was observed in the advanced tumor stage (Figure 3I,J). Furthermore, no significant differences were observed in the immunostaining of target proteins between the different prostate lobes.
4. Immunohistochemical Analysis of SDC Family Members and SDCBP in Human Prostate Tissues
Expression of SDC1 in normal prostate tissues (non-neoplastic tissue adjacent to the tumor), when positive, showed strong immunostaining in the basal cells of the epithelium and around the smooth muscle cells of the stroma and blood vessels (Figure 4A). In adenocarcinomas, SDC1 immunostaining was not present in some patient samples; however, it showed positive immunostaining in epithelial cells, in both Gleason 3 (low grade) and Gleason 4–5 adenocarcinomas (high grade) (Figure 4B,C). SDC2, SDC3 and SDC4 expression in normal tissue, when positive, were present at the basolateral membranes of luminal epithelial cells and in basal cells (Figure 4D,G,J). In adenocarcinomas, positive immunostaining for SDC2 was detected, with moderate immunostaining present throughout the cytoplasm of neoplastic epithelial cells of patients with Gleason 3 tumors (Figure 4E) and strong cytoplasmic and pericellular immunostaining in Gleason 4–5 tumors (Figure 4F). No immunostaining for SDC2 was observed in the glandular stroma.
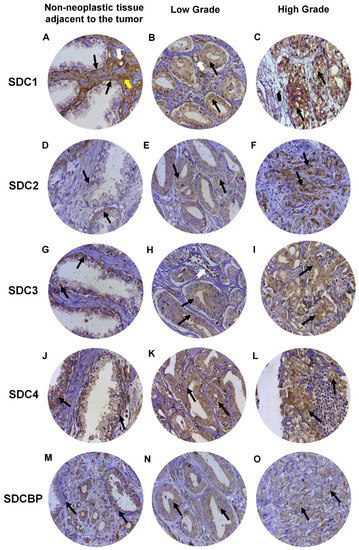
Figure 4. Representative images of immunohistochemical staining for SDC1, -2, -3, and -4, and SDCBP in adjacent non-neoplastic tissue (I), low-grade Gleason (Gleason grade 3), and high-grade Gleason (Gleason grades 4 or 5) from TMAs of human prostate samples. Images (A–C) show SDC1 staining. Images (D–F) show SDC2 staining. (G–I) show SDC3 staining. The (J–L) images show SDC4 staining. (M–O) images show SDCBP staining. Non-neoplastic tissue: (A,D,G,J,M). Low Grade: (B,E,H,I,K). High Grade: (C,F,I,L,O). Black arrows indicate positively stained cells. White arrows: blood vessels. Yellow arrow: smooth muscle cells. Final magnification: ×400.
In adenocarcinomas, when positive, SDC3 showed moderate immunostaining throughout the cytoplasm of neoplastic epithelial cells of patients with Gleason 3 tumors (Figure 4H) and weak cytoplasmic and pericellular immunostaining in Gleason 4–5 tumors (Figure 4I). Immunostaining of SDC3 was observed around blood vessels. Positive immunostaining was detected for SDC4 in adenocarcinomas, where strong immunostaining was observed throughout the cytoplasm of neoplastic epithelial cells of patients with Gleason 3 (Figure 4K) and Gleason 4–5 tumors (Figure 4L). Immunostaining of SDC4 was observed in the glandular stroma and cells of the immune system.
SDCBP expression in normal tissue, when positive, was present in the cytoplasm of luminal and basal epithelial cells (Figure 4M). Positive immunostaining was detected for SDCBP in adenocarcinomas. SDCBP presented moderate immunostaining throughout the cytoplasm of neoplastic epithelial cells of patients with Gleason 3 tumors (Figure 4N) and weak cytoplasmic and pericellular immunostaining in Gleason 4–5 tumors (Figure 4O). Immunostaining for SDCBP was present in the epithelial stroma of normal and tumor tissues.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168669
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278.
- Choudhury, A.D.; Eeles, R.; Freedland, S.J.; Isaacs, W.B.; Pomerantz, M.M.; Schalken, J.A.; Tammela, T.L.; Visakorpi, T. The role of genetic markers in the management of prostate cancer. Eur. Urol. 2012, 62, 577–587.
- Lu, Y.T.; Delijani, K.; Mecum, A.; Goldkorn, A. Current status of liquid biopsies for the detection and management of prostate cancer. Cancer Manag. Res. 2019, 11, 5271–5291.
- Campos-Fernández, E.; Barcelos, L.S.; de Souza, A.G.; Goulart, L.R.; Alonso-Goulart, V. Research landscape of liquid biopsies in prostate cancer. Am. J. Cancer Res. 2019, 9, 1309–1328.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55.
- Theocharis, A.D.; Skandalis, S.S.; Neill, T.; Multhaupt, H.A.; Hubo, M.; Frey, H.; Gopal, S.; Gomes, A.; Afratis, N.; Lim, H.C.; et al. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim. Biophys. Acta 2015, 1855, 276–300.
- Couchman, J.R. Syndecans: Proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 2003, 4, 926–937.
- Xian, X.; Gopal, S.; Couchman, J.R. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010, 339, 31–46.
- Gould, S.E.; Upholt, W.B.; Kosher, R.A. Syndecan 3: A member of the syndecan family of membrane-intercalated proteoglycans that is expressed in high amounts at the onset of chicken limb cartilage differentiation. Proc. Natl. Acad. Sci. USA 1992, 89, 3271–3275.
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans-key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41.
- Kim, C.W.; Goldberger, O.A.; Gallo, R.L.; Bernfield, M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 1994, 5, 797–805.
- Szatmári, T.; Ötvös, R.; Hjerpe, A.; Dobra, K. Syndecan-1 in cancer: Implications for cell signaling, differentiation, and prognostication. Dis. Markers 2015, 2015, 796052.
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685.
- Roucourt, B.; Meeussen, S.; Bao, J.; Zimmermann, P.; David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015, 25, 412–428.
- Cheng, B.; Montmasson, M.; Terradot, L.; Rousselle, P. Syndecans as cell surface receptors in cancer biology. a focus on their interaction with pdz domain proteins. Front. Pharmacol. 2016, 7, 10.
- Couchman, J.R.; Gopal, S.; Lim, H.C.; Norgaard, S.; Multhaupt, H.A. Fell-Muir lecture: Syndecans: From peripheral coreceptors to mainstream regulators of cell behaviour. Int. J. Exp. Pathol. 2015, 96, 1–10.
- Couchman, J.R. Syndecan-1 (CD138), Carcinomas and EMT. Int. J. Mol. Sci. 2021, 22, 4227.
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat. Res. Commun. 2021, 27, 100312.
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007.
- Meng, F.; Han, X.; Min, Z.; He, X.; Zhu, S. Prognostic signatures associated with high infiltration of Tregs in bone metastatic prostate cancer. Aging 2021, 13, 17442–17461.
- Kang, H.; Wu, Q.; Sun, A.; Liu, X.; Fan, Y.; Deng, X. Cancer cell glycocalyx and its significance in cancer progression. Int. J. Mol. Sci. 2018, 19, 2484.
- Loftus, P.G.; Watson, L.; Deedigan, L.M.; Camarillo-Retamosa, E.; Dwyer, R.M.; O’Flynn, L.; Alagesan, S.; Griffin, M.; O’Brien, T.; Kerin, M.J.; et al. Targeting stromal cell Syndecan-2 reduces breast tumour growth, metastasis and limits immune evasion. Int. J. Cancer 2021, 148, 1245–1259.
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol. Carcinog. 2016, 55, 1378–1386.
- Edwards, I.J. Proteoglycans in prostate cancer. Nat. Rev. Urol. 2012, 9, 196–206.
- Stepp, M.A.; Pal-Ghosh, S.; Tadvalkar, G.; Rajjoub, L.; Jurjus, R.A.; Gerdes, M.; Ryscavage, A.; Cataisson, C.; Shukla, A.; Yuspa, S.H. Loss of syndecan-1 is associated with malignant conversion in skin carcinogenesis. Mol. Carcinog. 2010, 49, 363–373.
- Parimon, T.; Brauer, R.; Schlesinger, S.Y.; Xie, T.; Jiang, D.; Ge, L.; Huang, Y.; Birkland, T.P.; Parks, W.C.; Habiel, D.M.; et al. Syndecan-1 Controls Lung Tumorigenesis by Regulating miRNAs Packaged in Exosomes. Am. J. Pathol. 2018, 188, 1094–1103.
- Wei, H.T.; Guo, E.N.; Dong, B.G.; Chen, L.S. Prognostic and clinical significance of syndecan-1 in colorectal cancer: A meta-analysis. BMC Gastroenterol. 2015, 15, 152.
- Palaiologou, M.; Delladetsima, I.; Tiniakos, D. CD138 (syndecan-1) expression in health and disease. Histol. Histopathol. 2014, 29, 177–189.
- Das, S.K.; Sarkar, D.; Emdad, L.; Fisher, P.B. MDA-9/Syntenin: An emerging global molecular target regulating cancer invasion and metastasis. Adv. Cancer Res. 2019, 144, 137–191.
- Pradhan, A.K.; Maji, S.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin/SDCBP: New insights into a unique multifunctional scaffold protein. Cancer Metastasis Rev. 2020, 39, 769–781.
This entry is offline, you can click here to edit this entry!
