Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dentistry, Oral Surgery & Medicine
Peri-implant diseases are inflammatory conditions affecting the soft and hard tissues around dental implants. This review will focus on the regenerative treatment of peri-implant osseous defects in order to provide some evidence that can aid clinicians in the approach to peri-implant disease treatment.
- peri-implant disease
- peri-implant mucositis
- peri-implantitis
1. Introduction
Due to the increasing number of dental implants placed every day in clinical practice, the biological complications related to these treatments are increasing. These complications range from inflammation and bleeding upon probing (BOP) to severe peri-implant bone resorption and implant failure [1]. Despite many investigations aimed at identifying the best approach to treat these conditions, there is still no universally recognized protocol to solve these complications successfully and predictably. The techniques available for the clinicians can be divided into non-surgical and surgical. Among the surgical options, the two main approaches are resective and regenerative treatments.
2. Peri Implant Diseases
Implant status ranges from clinical health to implant failure and loss. In particular, the implants are classified as health, peri-implant mucositis, and peri-implantitis. The review by Araujo and Lindhe in the 2017 World Workshop [2] described the healthy peri-implant mucosa as comprised of either a keratinized (masticatory mucosa) or non-keratinized epithelium (lining mucosa) with underlying connective tissue. The clinical criteria available to assess and diagnose implant conditions are the same used to assess and diagnose periodontal conditions around teeth: probing depth (PD), bleeding on probing (BOP), suppuration (SUP), and radiographs. These can identify an inflammatory status and periodontal/peri-implant bone loss. The same authors stated that peri-implant health requires the absence of clinical signs of inflammation such as erythema, swelling, and bleeding on probing (BOP). Radiographic evidence of crestal bone changes around implants is important when differentiating peri-implant health from disease. When inflammatory signs appear (BOP, erythema, soft tissue swelling), a diagnosis of peri-implant mucositis (PIM) can be done. When PIM is associated with the progressive loss of supporting peri-implant bone, the diagnosis of peri-implantitis (PI) is established [1]. It is generally accepted that 0.5 to 2 mm of crestal bone loss during healing is considered physiological bone remodeling following implant installation and initial loading [3]. However, any additional radiographic evidence of bone loss more than 2mm after the placement of the prosthetic supra-structure, would suggest PI [3]. Although the conversion from an inflammatory process identified as PIM to PI is not well understood, it is generally agreed that both diseases share the same infectious etiology through the development of biofilm [1]. If the lesion is left untreated, the inflammatory process can lead to progressive peri-implant bone loss and implant failure. In case of an absence of documentation from the time of implant placement to the time of disease manifestation, radiographic bone level ≥ 3 mm in combination with BOP and PD ≥ 6 mm is indicative of peri-implantitis [3] (Figure 1).
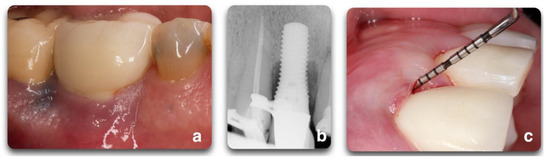
Figure 1. Representation of peri-implant clinical parameters that, associated, can lead to a diagnosis of peri-implantitis. In the sequence above, BOP/Suppuration (a), radiographic bone level ≥ 3 mm (b) in combination with PD ≥ 6 mm (c).
3. Peri-Implantitis Treatment Factors
The goal of peri-implantitis treatments is re-osseointegration or bone fill of the osseous defect to provide support to peri-implant soft tissue and thereby improve esthetic outcomes [4][5]; surgical regenerative approaches are indicated to achieve this goal. Nevertheless, these techniques are not always applicable due to varying defect morphologies and progressively advancing stages of the disease.
Re-osseointegration or regeneration, by definition, is an event that can only be assessed histologically in experimental models [4][5]. The efficacy of peri-implantitis treatment can vary depending on the outcome variables [5]. In fact, clinical protocols are limited to bone level assessments by radiographs and clinical variables (BOP, PD, REC), while experimental protocols also include histological evaluations regarding inflammation resolution and osseous defect repair [5]. Clinicians cannot truly assess re-osseointegration on their patients unless a histological specimen is harvested and analyzed. Radiographic investigations are commonly used as the other non-invasive tool to assess bone changes after therapy [6], although questions regarding their reliability have been reported [7][8] (Figure 2). The most accurate solution to identify defect configuration is by direct access during the surgical intervention [9].
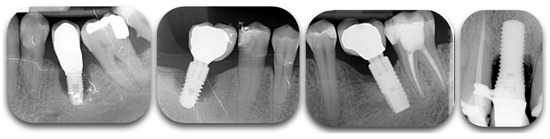
Figure 2. Radiographic films showing different degrees of peri-implant bone loss. The change of contrast can make the defect difficult to categorize.
3.1. Peri-Implant Defect Configuration
One of the most important factors related to the success of peri-implantitis regenerative procedures is the peri-implant bone defect configuration. These procedures are not aimed at addressing disease resolution but are an attempt to fill the defect created by the disease. The feasibility of this goal has been shown to be closely associated with the configuration of the peri-implant defect and the number of walls surrounding the lesion (Figure 3).
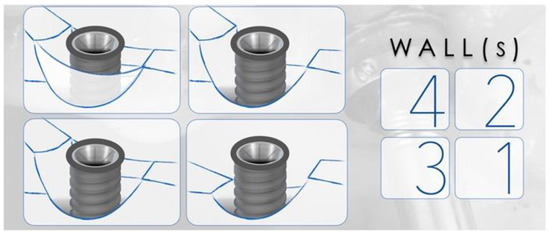
Figure 3. Representation of peri-implant defects according to the number of walls surrounding the lesion. The right panel corresponds to the number of walls present in the representations. The higher the number of walls, the higher is the positive outcomes of regenerative therapy.
Studies have described a relationship between the peri-implant defect morphology and the clinical success of peri-implantitis therapies [6][9][10]. Therefore, careful consideration of the defect morphology must be made when selecting a peri-implantitis intervention. In 2007, Schwarz et al. [11] proposed a classification of peri-implantitis defects verified through intra-surgical findings in humans and animals. The classification was based on intrabony features and horizontal bone loss, describing the absence of buccal and/or lingual walls, circumferential, and supra- or sub-crestal patterns (Figure 4). In 2019, Monje et al. [6] updated this classification by adding combined defects (Figure 5A).
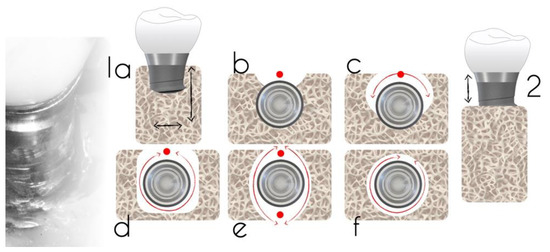
Figure 4. Defect classification according to an intra-operative assessment (adapted from Schwarz et al. [11]). Class 1a—facial view (a); Class 1a—occlusal view (b); Class 1b—buccal dehiscence + semicircular bone resorption to the middle of the implant body (c); Class 1c—buccal dehiscence + circular bone resorption with the lingual wall (d); Class 1d—circular bone resorption with lack of buccal and lingual walls (e); Class 1e—circular bone resorption with the presence of buccal and lingual wall; (f) Class 2: supra-crestal bone resorption.
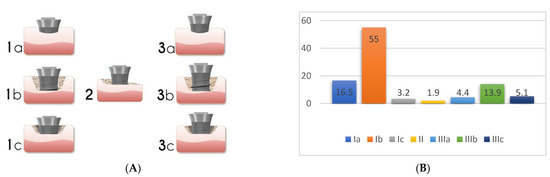
Figure 5. Peri-implant bone defect classification according to Monje et al. [6] (Adapted from original paper) (A). The figure is a representation of: Class I Infra-osseous defects, Class II Supra-crestal/Horizontal defects, and Class III Combined defects. Sub-classifications a (buccal dehiscence), b (2–3 wall defects), and c (circumferential configurations). (B) Prevalence of the different defect morphology types according to Monje et al. [6].
Prevalence data for the defect configurations seems to vary among studies. Schwarz et al., found circumferential defects to be the most prevalent (55.3%) [11]. In contrast, Garcia-Garcia et al. [12] found about 30% of defects to exhibit a circumferential configuration, while 25% included a buccal dehiscence combined with a circumferential defect. Aghazadeh et al. [9], on the other hand, found two-wall defects to be the most prevalent. Roccuzzo et al. [13] also introduced dissimilar data when exhibiting that one-third of cases (35.7%) had a semi-circumferential component combined with a buccal dehiscence. In a more recent study, Monje et al. [6] described three-wall defects as the most prevalent, followed by buccal dehiscence defects (Figure 5B).
It has been noted that the inconsistencies in these findings may be attributed to anatomical variations at implant placement. Schwarz et al. [11] described that the alveolar ridge width played a role in the number of bony walls formed in the future peri-implantitis defect. Moreover, the studies varied considerably in their anatomical location. For instance, most of the implants analyzed by Schwarz et al. were placed in the posterior region while other studies evaluated implants placed in the anterior and premolar regions [9]. Nonetheless, identifying the defect morphology yielded the opportunity to evaluate the predictability of various peri-implantitis treatments.
Understanding the peri-implant defect morphology is important because of its potential for determining the likelihood of regeneration therapy success [9]. For situations in which regenerative procedures are unlikely to produce favorable results, resective surgeries may offer more clinical benefits. Of all the defect configurations, circumferential defects (Ie) achieved the highest reduction in PD and clinical attachment level (CAL) [10], while class Ib and Ic defects showed the poorest. Class Ib defects were the most prevalent in many of the studies. Therefore, it is more common to see peri-implantitis defect morphologies that are poorly responsive to reconstructive therapies (Figure 6).
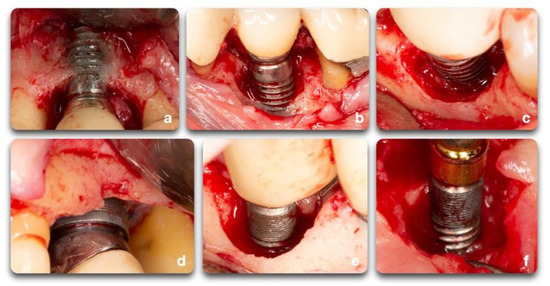
Figure 6. Clinical example of peri-implant bone defects; (a) facial dehiscence; (b,c) facial dehiscence and a semi-circumferential defect; (d) circumferential defect with a supra-crestal bone resorption; (e) circumferential defect; (f) semi-circumferential and supra-crestal bone defect.
3.2. Surface Decontamination
The implant surface holds great importance on the success and speed of osseointegration. Different implant brands are characterized by varying treatment surfaces and roughness [14]. The question on implant surface characteristics and healing following surgical therapy to treat peri-implantitis is not new. It is demonstrated that, as part of the regenerative procedure, implant decontamination is essential to obtain positive outcomes [15]. The consensus report from the 8th European Workshop on Periodontology [16] stated that implant surface decontamination is a critical component of surgical treatment. Implant decontamination is aimed at removing bacterial biofilm and resolving infection and inflammation, rendering the surface biocompatible and conducive to bone regeneration and possible re-osseointegration, or at least minimizing bacterial adhesion [4]. Various techniques have been proposed for implant surface decontamination after surgical exposure: mechanical, chemical, laser or photodynamic, or a combination of these [17] (Figure 7). The decontamination process presents multiple challenges. Besides the attempts to solve the infectious process, the implant threads and rough surfaces pose a significant obstacle to the mechanical cleansing that, if not optimal, can lead to the reestablishment of pathogenic microflora and persistence of pathology [17]. In advanced peri-implant defect lesions, surface decontamination alone will not adequately achieve bone regeneration. In these cases, filling the defect with graft materials and growth factors yields better outcomes. Investigations on surgical treatment for peri-implantitis range from in-vitro and animal studies to human clinical trials. Each of these fields provided important insight on outcomes and healing processes. Due to the limitations of each model, study design, length of treatment, materials, outcome measures, and the heterogeneity of data, it is difficult to compare outcome measurements [15][17][18][19][20].
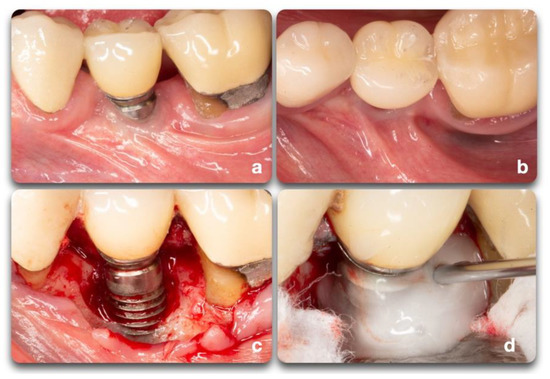
Figure 7. Clinical scenario representing a peri-implant defect affecting implant #20. A lack of keratinized mucosa (a) and frenum pull (b) could have played an important role in the defect shown (c). After mechanical debridement with curettes and titanium rotary brushes, the surface was treated with 24% EDTA gel to provide the chemical surface decontamination (d).
This entry is adapted from the peer-reviewed paper 10.3390/biology10080773
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. 1), S267–S290.
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Periodontol. 2018, 89 (Suppl. 1), S249–S256.
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S304–S312.
- Renvert, S.; Polyzois, I.; Maguire, R. Re-osseointegration on previously contaminated surfaces: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 216–227.
- Almohandes, A.; Carcuac, O.; Abrahamsson, I.; Lund, H.; Berglundh, T. Re-osseointegration following reconstructive surgical therapy of experimental peri-implantitis. A pre-clinical in vivo study. Clin. Oral Implant. Res. 2019, 30, 447–456.
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.-L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant Dent. Relat. Res. 2019, 21, 635–643.
- Pazzaglia, U.E.; Bernini, F.; Zatti, G.; Di Nucci, A. Histology of the metal-bone interface: Interpretation of plastic embedded slides. Biomaterials 1994, 15, 273–277.
- Sykaras, N.; Iacopino, A.M.; Triplett, R.G.; Marker, V.A. Effect of Recombinant Human Bone Morphogenetic Protein-2 on the Osseointegration of Dental Implants: A Biomechanics Study. Clin. Oral Investig. 2004, 8, 196–205.
- Aghazadeh, A.; Persson, R.G.; Renvert, S. Impact of bone defect morphology on the outcome of reconstructive treatment of peri-implantitis. Int. J. Implant Dent. 2020, 6, 33.
- Schwarz, F.; Sahm, N.; Mihatovic, I.; Golubovic, V.; Becker, J. Surgical therapy of advanced ligature-induced peri-implantitis defects: Cone-beam computed tomographic and histological analysis. J. Clin. Periodontol. 2011, 38, 939–949.
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implant. Res. 2007, 18, 161–170.
- García-García, M.; Mir-Mari, J.; Benic, G.I.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy of periapical radiography in assessing bone level in implants affected by peri-implantitis: A cross-sectional study. J. Clin. Periodontol. 2016, 43, 85–91.
- Roccuzzo, M.; Pittoni, D.; Roccuzzo, A.; Charrier, L.; Dalmasso, P. Surgical treatment of peri-implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7-Year-results. Clin. Oral Implant. Res. 2017, 28, 1577–1583.
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40.
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis-Consensus report of working group 4. Int. Dent. J. 2019, 69 (Suppl. 2), 18–22.
- Sanz, M.; Chapple, I.L. Clinical research on peri-implant diseases: Consensus report of Working Group 4. J. Clin. Periodontol 2012, 39 (Suppl. 12), 202–206.
- Koo, K.T.; Khoury, F.; Keeve, P.L.; Schwarz, F.; Ramanauskaite, A.; Sculean, A.; Romanos, G. Implant Surface Decontamination by Surgical Treatment of Periimplantitis: A Literature Review. Implant Dent. 2019, 28, 173–176.
- Ramanauskaite, A.; Obreja, K.; Sader, R.; Khoury, F.; Romanos, G.; Koo, K.T.; Keeve, P.L.; Sculean, A.; Schwarz, F. Surgical Treatment of Periimplantitis With Augmentative Techniques. Implant Dent. 2019, 28, 187–209.
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345.
- Renvert, S.; Polyzois, I.N. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol. 2000 2015, 68, 369–404.
This entry is offline, you can click here to edit this entry!
