Hepatocellular carcinoma (HCC) is one of the deadliest human cancers. Activating mutations in the telomerase reverse transcriptase (TERT) promoter (TERTp) and CTNNB1 gene encoding β-catenin are widespread in HCC (~50% and ~30%, respectively). TERTp mutations are predicted to increase TERT transcription and telomerase activity.
- hepatocellular carcinoma
- TERT
- TERT promoter
- β-catenin
1. Introduction
2. Telomeres, Telomerase, and TERT
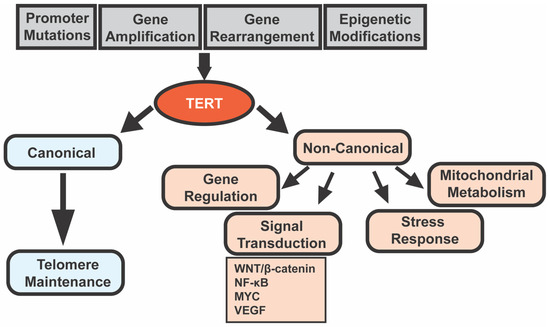
3. Telomerase and Cancer
| Gene (Animal) | Site Specificity | Expression * | Result ** | Ref. |
|---|---|---|---|---|
| Tert (mouse) | Thymocytes and peripheral T cells | + | ↑ T-Cell Lymphomas | [16] |
| Tert (mouse) | Basal keratinocytes | + | ↑ skin papillomas (DMBA + TPA induction) | [17] |
| Tert (mouse) | Whole body | + | ↑ mammary tumors in aged females | [18] |
| tert and terc (zebrafish) | Neural progenitor cells | + | ↓ aggressiveness of RAS-mediated brain tumors | [27] |
| Tert (mouse) | Whole body | − | Delayed onset of lymphomas in EμMYC mice | [20] |
| Tert (mouse) | Whole body | − | Delayed onset of mammary tumors in PyMT mice | [19] |
| Tert (mouse) | Whole body | − | ↓ HCC “initiation foci” (CCl4 induction) | [21] |
| Terc (mouse) | Whole body | − | ↑ HCC “initiation foci” but ↓ HCC progression (uPA, CCl4 or DEN induction) | [22] |
| Terc (mouse) | Whole body | − | ↑ tumors (lymphomas, teratocarcinomas, HCC, squamous cell carcinoma) | [26] |
| Terc (mouse) | Whole body | − | ↓ skin papillomas (DMBA + TPA induction) | [28] |
| Terc (mouse) | Whole body | − | ↑ epithelial cancers in TP53−/− mice | [29] |
| Terc (mouse) | Whole body | − | ↑ adenoma initiation but ↓ progression in ApcMin mice | [30] |
| terthu3430 (zebrafish) | Whole body | − | Earlier onset of tumors (germ cell tumors, hematopoietic neoplasms, HCA, etc.) | [23] |
| terthu3430 (zebrafish) | Whole body | − | ↑ tumor incidence and aggressiveness of melanoma model *** | [31] |
*: +, gene overexpression; −, gene knockout or knockdown. **: ↑, increased; ↓, decreased. *** mitfa: HRAS (gives rise to melanomas) blastula cells transplanted into tert−/− casper embryos. Abbreviations: DMBA, 7,12-dimethylbenz[a]anthracene; TPA, 12-o-tetradecanoylphorbol 13-acetate; PyMT, polyomavirus middle T oncogene; CCl4, carbon tetrachloride; uPA, urokinase plasminogen activator; DEN, diethylnitrosamine; HCA, hepatocellular adenoma; Apc, adenomatous polyposis coli; ApcMin, multiple intestinal neoplasia (mutant) allele of Apc gene; and terthu3430, tert mutant line (allele hu3430) with a non-sense mutation resulting in a premature stop codon in exon 2 of tert gene.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13164202
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604.
- Lee, J.-S. The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 220–229.
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72.
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-Catenin Signaling Regulates Telomerase in Stem Cells and Cancer Cells. Science 2012, 336, 1549.
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218.
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511.
- Totoki, Y.; Tatsuno, K.; Covington, K.R.; Ueda, H.; Creighton, C.J.; Kato, M.; Tsuji, S.; Donehower, L.A.; Slagle, B.L.; Nakamura, H.; et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014, 46, 1267–1273.
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241.
- Heidenreich, B.; Kumar, R. TERT promoter mutations in telomere biology. Mutat. Res. Rev. Mutat. Res. 2017, 771, 15–31.
- Zhang, Q.; Kim, N.-K.; Feigon, J. Architecture of human telomerase RNA. Proc. Natl. Acad. Sci. USA 2011, 108, 20325–20332.
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7, 332.
- Romaniuk, A.; Paszel-Jaworska, A.; Totoń, E.; Lisiak, N.; Hołysz, H.; Królak, A.; Grodecka-Gazdecka, S.; Rubiś, B. The non-canonical functions of telomerase: To turn off or not to turn off. Mol. Biol. Rep. 2019, 46, 1401–1411.
- Yan, J.; Zhou, Y.; Chen, D.; Li, L.; Yang, X.; You, Y.; Ling, X. Impact of mitochondrial telomerase over-expression on drug resistance of hepatocellular carcinoma. Am. J. Transl. Res. 2015, 7, 88–99.
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69.
- Yuan, X.; Dai, M.; Xu, D. TERT promoter mutations and GABP transcription factors in carcinogenesis: More foes than friends. Cancer Lett. 2020, 493, 1–9.
- Canela, A.; Martín-Caballero, J.; Flores, J.M.; Blasco, M.A. Constitutive expression of tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol. Cell Biol. 2004, 24, 4275–4293.
- González-Suárez, E.; Samper, E.; Ramírez, A.; Flores, J.M.; Martín-Caballero, J.; Jorcano, J.L.; Blasco, M.A. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001, 20, 2619–2630.
- Artandi, S.E.; Alson, S.; Tietze, M.K.; Sharpless, N.E.; Ye, S.; Greenberg, R.A.; Castrillon, D.H.; Horner, J.W.; Weiler, S.R.; Carrasco, R.D.; et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8191–8196.
- Khattar, E.; Kumar, P.; Liu, C.Y.; Akıncılar, S.C.; Raju, A.; Lakshmanan, M.; Maury, J.J.P.; Qiang, Y.; Li, S.; Tan, E.Y.; et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J. Clin. Investig. 2016, 126, 4045–4060.
- Koh, C.M.; Khattar, E.; Leow, S.C.; Liu, C.Y.; Muller, J.; Ang, W.X.; Li, Y.; Franzoso, G.; Li, S.; Guccione, E.; et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Investig. 2015, 125, 2109–2122.
- Farazi, P.A.; Glickman, J.; Horner, J.; DePinho, R.A. Cooperative Interactions of p53 Mutation, Telomere Dysfunction, and Chronic Liver Damage in Hepatocellular Carcinoma Progression. Cancer Res. 2006, 66, 4766.
- Farazi, P.A.; Glickman, J.; Jiang, S.; Yu, A.; Rudolph, K.L.; DePinho, R.A. Differential Impact of Telomere Dysfunction on Initiation and Progression of Hepatocellular Carcinoma. Cancer Res. 2003, 63, 5021.
- Carneiro, M.C.; Henriques, C.M.; Nabais, J.; Ferreira, T.; Carvalho, T.; Ferreira, M.G. Short Telomeres in Key Tissues Initiate Local and Systemic Aging in Zebrafish. PLoS Genet. 2016, 12, e1005798.
- Marzec, P.; Armenise, C.; Pérot, G.; Roumelioti, F.-M.; Basyuk, E.; Gagos, S.; Chibon, F.; Déjardin, J. Nuclear-receptor-mediated telomere insertion leads to genome instability in ALT cancers. Cell 2015, 160, 913–927.
- Cayuela, M.L.; Claes, K.B.M.; Ferreira, M.G.; Henriques, C.M.; van Eeden, F.; Varga, M.; Vierstraete, J.; Mione, M.C. The Zebrafish as an Emerging Model to Study DNA Damage in Aging, Cancer and Other Diseases. Front. Cell Dev. Biol. 2019, 6, 178.
- Rudolph, K.L.; Chang, S.; Lee, H.-W.; Blasco, M.; Gottlieb, G.J.; Greider, C.; DePinho, R.A. Longevity, Stress Response, and Cancer in Aging Telomerase-Deficient Mice. Cell 1999, 96, 701–712.
- Idilli, A.I.; Cusanelli, E.; Pagani, F.; Berardinelli, F.; Bernabé, M.; Cayuela, M.L.; Poliani, P.L.; Mione, M.C. Expression of tert Prevents ALT in Zebrafish Brain Tumors. Front. Cell Dev. Biol. 2020, 8, 65.
- González-Suárez, E.; Samper, E.; Flores, J.M.; Blasco, M.A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat. Genet. 2000, 26, 114–117.
- Artandi, S.E.; Chang, S.; Lee, S.-L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645.
- Rudolph, K.L.; Millard, M.; Bosenberg, M.W.; DePinho, R.A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 2001, 28, 155–159.
- Lex, K.; Maia Gil, M.; Lopes-Bastos, B.; Figueira, M.; Marzullo, M.; Giannetti, K.; Carvalho, T.; Ferreira, M.G. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 15066.
