Breast cancer persists as a diffuse source of cancer despite persistent detection and treatment. Flavonoids, a type of polyphenol, appear to be a productive option in the treatment of breast cancer, because of their capacity to regulate the tumor related functions of class of compounds. Plant polyphenols are flavonoids that appear to exhibit properties which are beneficial for breast cancer therapy. Numerous epidemiologic studies have been performed on the dynamic effect of plant polyphenols in the prevention of breast cancer. There are also subclasses of flavonoids that have antioxidant and anticarcinogenic activity. These can regulate the scavenging activity of reactive oxygen species (ROS) which help in cell cycle arrest and suppress the uncontrolled division of cancer cells. Numerous studies have also been performed at the population level, one of which reported a connection between cancer risk and intake of dietary flavonoids. Breast cancer appears to show intertumoral heterogeneity with estrogen receptor positive and negative cells.
- flavonoids
- nanoparticles
- breast cancer
- drug delivery system
- cancer therapeutics
- anticancer
- phytochemicals
- apoptosis
- epidemiological study
1. Introduction
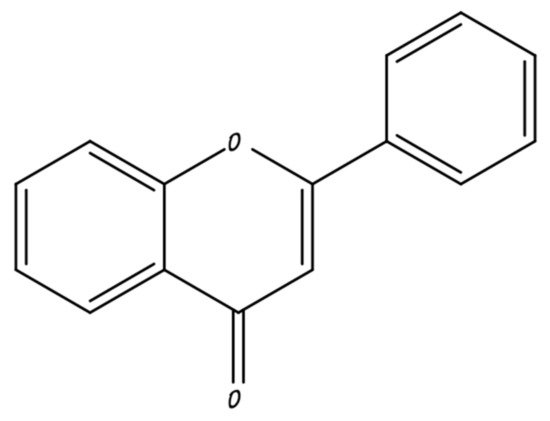
2. Flavonoids Used in Breast Cancer
| NO. | FLAVONOIDS | CONSTITUENTS |
|---|---|---|
| 1 | FLAVONES | Consist of apigenin, tangeretin, luteolin, chrysin [29] |
| 2 | FLAVONOLS | Consist of quercetin, isorhamnetin, myricetin, kaempferol [30] |
| 3 | FLAVONOIDS | Consist of naringenin, eriodictyol, hesperidin |
| 4 | CATECHINS | Consist of epicatechin, epigallocatechin gallate, (−)-epicatechingallate (ECG), (−)-epigallocatechin found in tea leaves |
| 5 | ISOFLAVONES | Consist of biochanin A, phytoestrogens, genistein, formononetin, daidzein |
| 6 | ANTHOCYANIDINS | Pelargonidin, cyanidin, malvidin, delphinidin, peonidin, petunidin |
| 7 | PROCYANIDINS | These are the oligomers of epicatechin, gallic acid, esters and catechin |
| 8 | LIGNANS | Plant components absorbed in gut to form phytoestrogens, enterolactone, enterodiol |
| 9 | FLAVAN-3-OLS | Consist of catechin, epicatechin, epigallocatechin, theaflavin-3-gallate, theaflavin-3,3′-digallic acid [29][31] |
2.1. Flavones
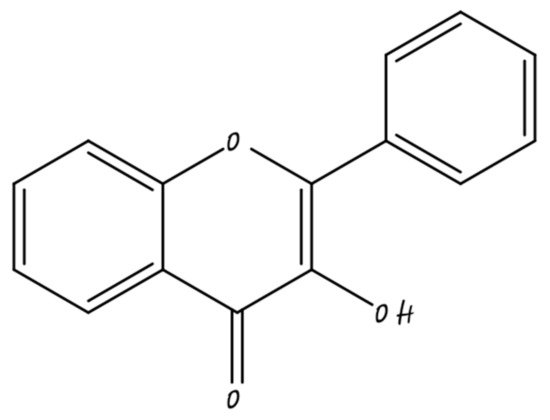
2.2. Flavonols
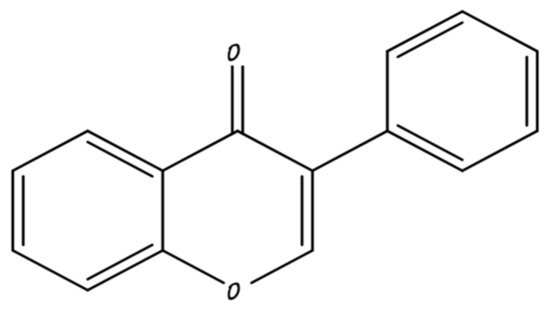
2.3. Catechins
2.4. Isoflavones
2.5. Anthocyanidins
- (1)
-
Signal pathway inhibition for blocking of signal transduction.
- (2)
-
Regulation of antioncogenes.
- (3)
-
By acting on beta-catechin, Notch pathway.
3. Nano-Based Formulation of Phyto-Bioactive Used in Breast Cancer Prevention
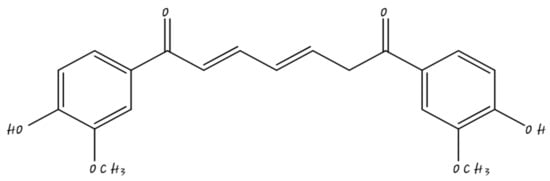
3.1. Nano-Curcumin
3.2. Nano-Epigallocatechin-3-Gallate
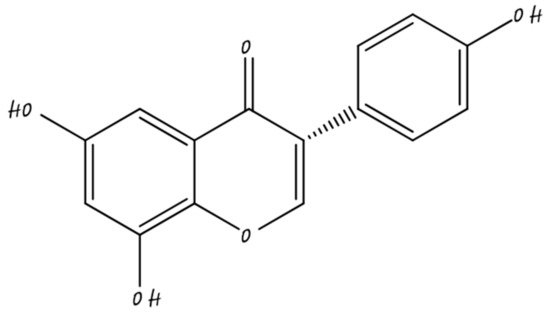
3.3. Nano-Naringenin
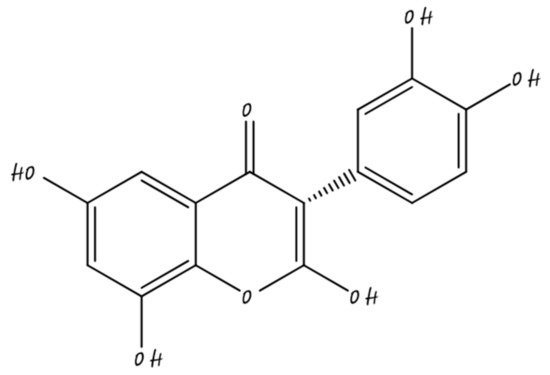
3.4. Nano-Quercetin
3.5. Nano-Resveratrol
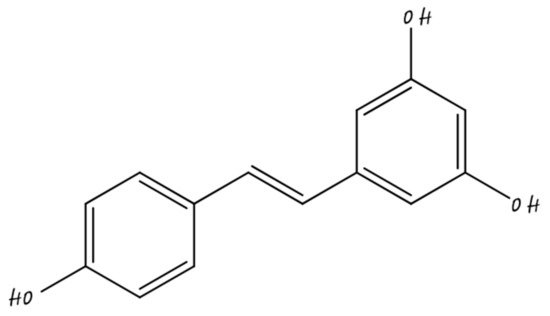
3.6. Nano-Apigenin
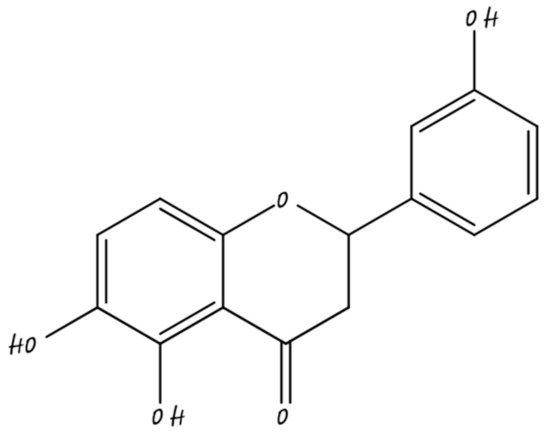
3.7. Nano-Silibrin
3.8. Nano-Genistein
| NO. | NANOFORMULATION | MECHANISM OF ACTION |
|---|---|---|
| 1 | NANO-CURCUMIN | Use of curcumin in targeting and prevention of different aging related pathological situations has been recorded [61] |
| 2 | NANO-EPIGALLOCATECHIN-3-GALLATE | Delivery of docetaxel present in EGCG-nanoethosomes transdermal diminish the volume of tumor [68] |
| 3 | NANO-NARINGENIN | Works by inhibiting both P13K and MAPK paths also restricts ER alpha to lessen the proliferation of tam-RMC cells [72] |
| 4 | NANO-QUERCETIN | It acts as growth inhibitor of MCF-7 cell lines |
| 5 | NANO-RESVERATROL | Combinational effect of resveratrol and Beta-glucan potentiates the composition on gene countenance (such as cdc42, NF-KB2 and BCL-2) in breast cancer cells [78] |
| 6 | NANO-APIGENIN | Biosensors used in photothermal therapy and have acquired implementation in treatment of cancer [79] |
| 7 | NANO-SILIBRIN | Silibrin loaded nanoparticles (SLNs) contain inhibitory effects on conquering of MDA-MB-231 cells |
| 8 | NANO-GENISTEIN | Responsible for the arrest of growth of MCF-4 cells at M/G2 phase and lower at proliferative S-phase [83] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175163
References
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137.
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211.
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–4.
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crop. Prod. 2015, 76, 582–589.
- Theodoratou, E.; Timofeeva, M.; Li, X.; Meng, X.; Ioannidis, J.P. Nature, nurture, and cancer risks: Genetic and nutritional contributions to cancer. Annu. Rev. Nutr. 2017, 37, 293–320.
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28.
- He, X.; Xue, M.; Jiang, S.; Li, W.; Yu, J.; Xiang, S. Fucoidan promotes apoptosis and inhibits emt of breast cancer cells. Biol. Pharm. Bull. 2019, 42, 442–447.
- Beatrice Magne Nde, C.; Zingue, S.; Winter, E.; Beatriz Creczynski-Pasa, T.; Michel, T.; Fernandez, X.; Njamen, D.; Clyne, C. Flavonoids, breast cancer chemopreventive and/or chemotherapeutic agents. Curr. Med. Chem. 2015, 22, 3434–3446.
- Schick, J.; Ritchie, R.P.; Restini, C. Breast Cancer Therapeutics and Biomarkers: Past, Present, and Future Approaches. Breast Cancer Basic Clin. Res. 2021, 15, 1178223421995854.
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidem. Biomar. 2001, 10, 53–58.
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurfs 2-dependent ubiquitin proteasome pathway in TGFβ receptor degradation. Oncotarget 2014, 5, 7870.
- Aune, D.; Chan, D.S.; Vieira, A.R.; Rosenblatt, D.N.; Vieira, R.; Greenwood, D.C.; Norat, T. Fruits, vegetables and breast cancer risk: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 134, 479–493.
- Reiss, R.; Johnston, J.; Tucker, K.; DeSesso, J.M.; Keen, C.L. Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food Chem. Toxicol. 2012, 50, 4421–4427.
- Zhang, C.X.; Ho, S.C.; Chen, Y.M.; Lin, F.Y.; Fu, J.H.; Cheng, S.Z. Meat and egg consumption and risk of breast cancer among Chinese women. Cancer Causes Control 2009, 20, 1845–1853.
- Butler, L.M.; Wu, A.H.; Wang, R.; Koh, W.P.; Yuan, J.M.; Yu, M.C. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am. J. Clin. Nutr. 2010, 91, 1013–1019.
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Medical Science Monitor. Int. J. Clin. Exp. Med. 2018, 24, 412.
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457.
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New frontier for immune-regulation and breast cancer control. Antioxidants 2019, 8, 103.
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 19, 1–13.
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387.
- De Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196.
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689.
- Lee, T.J.; Kim, O.H.; Kim, Y.H.; Lim, J.H.; Kim, S.; Park, J.W.; Kwon, T.K. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006, 240, 234–242.
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184.
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171.
- Serra, D.; Parris, C.R.; Carper, E.; Homel, P.; Fleishman, S.B.; Harrison, L.B.; Chadha, M. Outcomes of guided imagery in patients receiving radiation therapy for breast cancer. Clin. J. Oncol. Nurs. 2012, 16, 617.
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Raina, R.; Hussain, A.; Sharma, R. Molecular insight into apoptosis mediated by flavones in cancer. World Acad. Sci. J. 2020, 2, 1.
- Xian, D.; Guo, M.; Xu, J.; Yang, Y.; Zhao, Y.; Zhong, J. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. Commun. Free. Radic. Res. 2021, 26, 134–146.
- Wruck, C.J.; Claussen, M.; Fuhrmann, G.; Römer, L.; Schulz, A.; Pufe, T.; Wätzig, V.; Peipp, M.; Herdegen, T.; Götz, M.E. Luteolin protects rat PC 12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap-Nrf2-ARE pathway. Neuropsychiatr. Disord. Int. Approach 2007, 25, 57–67.
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645.
- Braakhuis, A.J.; Campion, P.; Bishop, K.S. Reducing breast cancer recurrence: The role of dietary polyphenolics. Nutrients 2016, 8, 547.
- Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, flavonoid subclasses, and esophageal cancer risk: A meta-analysis of epidemiologic studies. Nutrients 2016, 8, 350.
- Sudan, S.; Rupasinghe, H.V. Quercetin-3-O-glucoside induces human DNA topoisomerase II inhibition, cell cycle arrest and apoptosis in hepatocellular carcinoma cells. Anticancer Res. 2014, 34, 1691–1699.
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342.
- Nikolaou, K.C.; Talianidis, I. Hepatic cancer stem cells may arise from adult ductal progenitors. MCO 2016, 3, e1021946.
- Noolu, B.; Gugulothu, R.; Bhat, M.; SYH Qadri, S.; Sudhakar Reddy, V.; Bhanuprakash Reddy, G.; Ismail, A. In vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Curr. Med. Chem. Anticancer Agents 2016, 16, 1605–1614.
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277.
- Iwashina, T. Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull. Natl. Mus. Nat. Sci. 2013, 39, 25–51.
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506.
- Akhtar Siddiqui, J.; Singh, A.; Chagtoo, M.; Singh, N.; Madhav Godbole, M.; Chakravarti, B. Phytochemicals for breast cancer therapy: Current status and future implications. Curr. Cancer Drug Targets 2015, 15, 116–135.
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Li, Q.S.; Liang, Y.R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458.
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. Int. J. Food Prop. 2008, 11, 258–272.
- Li, M.; Tse, L.A.; Chan, W.C.; Kwok, C.H.; Leung, S.L.; Wu, C.; Yu, W.C.; Yu, I.T.; Yu, C.H.; Wang, F.; et al. Evaluation of breast cancer risk associated with tea consumption by menopausal and estrogen receptor status among Chinese women in Hong Kong. Cancer Epidemiol. 2016, 40, 73–78.
- Suzuki, Y.; Tsubono, Y.; Nakaya, N.; Koizumi, Y.; Tsuji, I. Green tea and the risk of breast cancer: Pooled analysis of two prospective studies in Japan. Br. J. Cancer 2004, 90, 1361–1363.
- Zhang, M.; Holman, C.D.; Huang, J.P.; Xie, X. Green tea and the prevention of breast cancer: A case–control study in Southeast China. Carcinogenesis 2007, 28, 1074–1078.
- Yiannakopoulou, E.C. Effect of green tea catechins on breast carcinogenesis: A systematic review of in-vitro and in-vivo experimental studies. Eur. J. Cancer Prev. 2014, 23, 84–89.
- Alshatwi, A.A. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 1–9.
- Kucuk, O. Soy foods, isoflavones, and breast cancer. Cancer 2017, 123, 1901–1903.
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S.
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754.
- Badger, T.M.; Ronis, M.J.; Hakkak, R.; Rowlands, J.C.; Korourian, S. The health consequences of early soy consumption. J. Nutr. 2002, 132, 559S–565S.
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076.
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243.
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336.
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290.
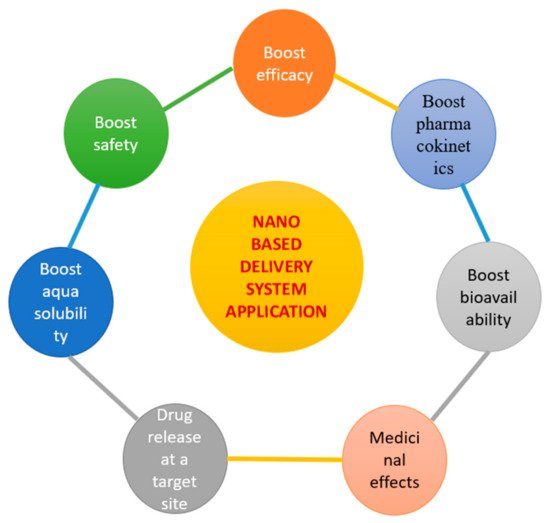
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33.
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of natural antioxidants: An anti-aging perspective. Front. Bioeng. Biotechnol. 2020, 7, 447.
- Dhupal, M.; Chowdhury, D. Phytochemical-Based Nanomedicine for Advanced Cancer Theranostics: Perspectives on Clinical Trials to Clinical Use. Int. J. Nanomed. 2020, 15, 9125.
- Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic potential and recent advances of curcumin in the treatment of aging-associated diseases. Molecules 2018, 23, 835.
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545.
- Mohammad Hossein, K.; Amin, D.; Afsaneh, N.; Alireza, H.; Sima, H.; Fereidon, N.; Sepideh, K.; Maliheh, E.; Mehrdad, H.; Hossein, D. Analysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iran. J. Pharm. Res. 2016, 15, 231.
- Dobrzyńska, M.; Napierała, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268.
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307.
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Devel. Ther. 2016, 10, 3519.
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin gallate (EGCG)-nanoerythrosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31.
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented anticancer activity of naringenin-loaded TPGS polymeric nanosuspension for drug resistive MCF-7 human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761.
- Noori, S.; Tavirani, M.R.; Deravi, N.; Rabbani, M.I.; Zarghi, A. Naringenin enhances the anti-cancer effect of cyclophosphamide against MDA-MB-231 breast cancer cells via targeting the STAT3 signaling pathway. Iran. J. Pharm. Res. IJPR 2020, 19, 122.
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291.
- Ramos, J.; Hatkevich, T.; Eanes, L.; Santos-Sanchez, I.; Patel, Y.M. Naringenin inhibits proliferation and survival of tamoxifen-resistant breast cancer cells. In Breast Cancer—From Biology to Medicine; IntechOpen: London, UK, 2017.
- Niazaand, F.; Razizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114.
- Kim, W.K.; Bang, M.H.; Kim, E.S.; Kang, N.E.; Jung, K.C.; Cho, H.J.; Park, J.H. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 2005, 16, 155–162.
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463.
- Singh, R.; Kumari, P.; Kumar, S. Nanotechnology for enhanced bioactivity of bioactive phytomolecules. In Nutrient Delivery; Academic Press: Amsterdam, The Netherlands, 2017; pp. 413–456.
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front. Pharmacol. 2019, 9, 1534.
- Shindikar, A.; Singh, A.; Nobre, M.; Kirolikar, S. Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J. Oncol. Res. 2016, 1–13.
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm. Cancer 2012, 3, 160–171.
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50.
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327.
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446.
- Liu, Y.; Xie, X.; Hou, Z.; Shen, J.; Shi, J.; Chen, H.; He, Y.; Wang, Z.; Feng, N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J. Nanobiotechnol. 2020, 18, 83.
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30.
- Ranganatha, S.; Shruthi, S.D.; Govindappa, M.; Ramachandra, Y.L. In silico studies of NF-κB protein as anti-cancer and anti-inflammatory target. J. Comput. Methods Mol. Des. 2013, 3, 26–33.
- Chen, W.F.; Huang, M.H.; Tzang, C.H.; Yang, M.; Wong, M.S. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim. Biophys. Acta Mol. Basis Dis. 2003, 1638, 187–196.
- Pool, H.; Campos-Vega, R.; Herrera-Hernández, M.G.; García-Solis, P.; García-Gasca, T.; Sánchez, I.C.; Luna-Bárcenas, G.; Vergara-Castañeda, H. Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. Am. J. Transl. Res. 2018, 10, 2306.
