ASX 主要由各种藻类、细菌和真菌生物合成,供海洋动物食用,如鲑鱼、鳟鱼、螃蟹、龙虾和虾。据报道,叶绿藻类雨生红球藻在自然界中积累了最高水平的 ASX。此外,ASX 主要以单酯或二酯的形式存在,可在小肠中水解,促进其吸收和运输到血浆和红细胞中,并有效地在皮肤中积累,使人体皮肤成为主要的生理目标。
- astaxanthin
- skin ageing
- antioxidant
- anti-inflammation
一、简介
Astaxanthin (ASX) is a xanthophyll carotenoid, chemically known as 3,3′-dihydroxy-β,β-carotene-4,4′-dione. It was originally isolated from lobster by Kuhn in 1938 [1]. Its characteristic bright red-orange colour occurs in salmon flesh, thus it is widely used as a colourant in aquaculture feeds [2]. ASX is primarily biosynthesised by various algae, bacteria and fungi and is consumed by marine animals, such as salmon, trout, crab, lobster and shrimp [3]. The chlorophyte alga, Haematococcus Pluvialis, is reported to accumulate the highest levels of ASX in nature [4]. ASX occurs predominantly as monoesters or diesters that may be hydrolysed in the small intestine, which facilitates its absorption and transportation into plasma and erythrocytes [5] and accumulation in the skin effectively [6], making human skin the major physiological target. Skin ageing is becoming a global challenge due to the rapidly increasing human lifespan around the world and intensive ultra-violet rays contributing to the destruction of ozone layers [7]. This finding attracts a great deal of scientific interest to investigate if ASX could prevent or even slow down skin ageing, and have a promising cosmeceutical potential.
The skin comprises epidermis, dermis and hypodermis, which form the protectively outermost barrier against external environmental stresses such as repeated sun UV ray exposure, microorganisms or pathogens invasion, physicochemical agents and excessive transpiration of internal moisture [8]. Photo ageing accounts for most age-related changes in skin appearance, which is caused by the superpositioning of chronic UV-induced deteriorations on the intrinsic ageing process [9]. Aged skin is characterized by skin dryness, deep wrinkling, laxity and increasing transepidermal water loss (TEWL) (the loss of water passing from the inside body through the epidermis) and epidermal barrier dysfunctions [10].
UV radiation comprises three types: UVA, UVB and UVC. UVC is filtered out by atmospheric ozone for the most part, while both UVA and UVB can cause a biological change in the skin [6]. UVA, which contributes up to 95% of the total UV exposure, results in skin photoaging by penetrating skin dermis and degrading the epidermal layer supporters: dermal collagen and elastin which are produced by the fibroblasts and are responsible for skin strength and elasticity, respectively [11]. UVB mainly affects the epidermis and epidermal keratinocytes that could communicate with dermal fibroblasts to facilitate relief to exogenous and endogenous damages [8]. UVB could also damage cellular macromolecules (nucleic acids, lipid and protein) and induce generations of high levels of ROS, stimulating chronic inflammation (See Figure 1 ) [6]. Moreover, intrinsic skin ageing rapidly accelerates especially after the age of 30 [12], because cell metabolic activities, such as the capacity of endogenous antioxidants enzymes (i.e., glutathione peroxide (GPx) from epidermis) for the elimination of the oxidized biomolecules, are reduced as with age, promoting an accumulation of intracellular reactive oxygen species (ROS) and inflammatory responses [13]. Meanwhile, the gradually disrupted epidermal barrier accelerates skin ageing [8] owing to the loss of functions of several important factors, such as proteases, proteases inhibitors, corneocytes (consist of dead epidermal cells) and lipids [8].
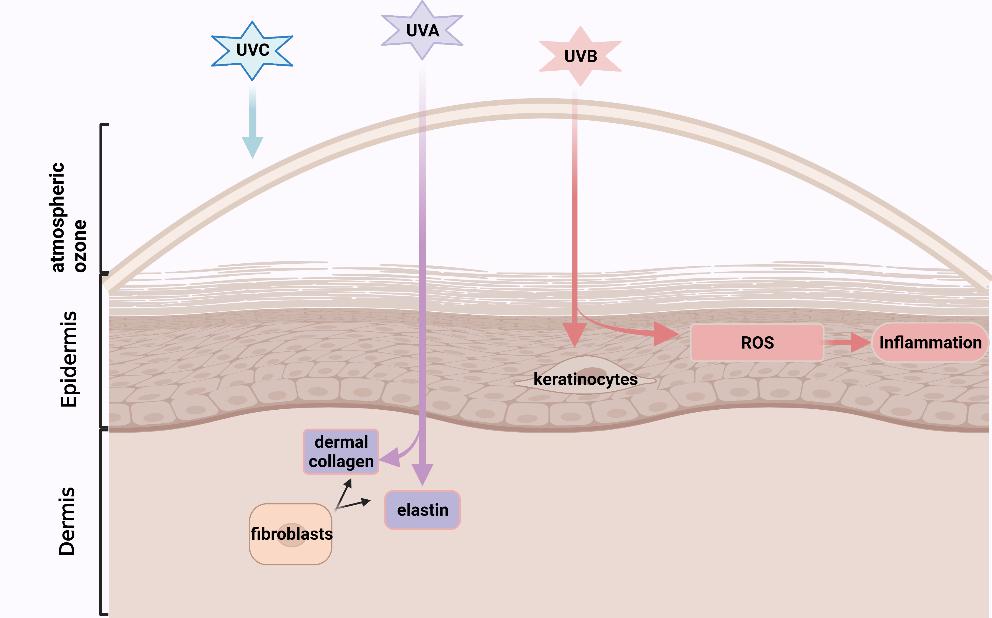
Figure 1. The mechanisms that UV-rays promote skin photoaging (created with BioRender.com.
https://app.biorender.com/illustrations/61123dbdedaefd00a5e711d7. Accessed on 12 August 2021)
ASX is structurally similar to β-carotene (See Figure 2 ) but has 40 times stronger antioxidant activity, as its polar ionone rings on both ends can quench free radicals and other reactive oxygen species (ROS), and the thirteen conjugated double, polyunsaturated bonds can remove high-energy electrons [14]. Its amphipathic structure with polar-nonpolar-polar characteristics allows ASX to be inserted into the bilayers of cell membranes, confines lipoperoxidation promoters to penetrate across the lipid bilayer and thus reduces peroxidation-caused damages [15]. Dietary ASX supplementation could lower wrinkle formations, decreased TEWL and maintain epidermal barrier functions significantly in the dorsal skin of HR-1 hairless mice under UVA exposure by its antioxidative effects, as compared to the control group [6]. In addition, ASX also has potential anti-inflammation effects by inhibiting inflammatory mediators [16]. Atopic dermatitis (AD) is a disease that is commonly found in aged people with reduced skin barrier functions due to slowing metabolic activity [17]. ASX cream could improve the development of phthalic anhydride (PA)-induced AD in HR-1 hairless mice by inhibiting the releases of various inflammatory cytokines [18]. However, the beneficial effects found in animal studies cannot be directly translated into protective effects in humans partially due to the different bioavailability of ASX across species. This review aims to conduct a systemic review and meta-analysis to evaluate the effects of ASX on human skin ageing, based on currently published trials and highlight future research directions.
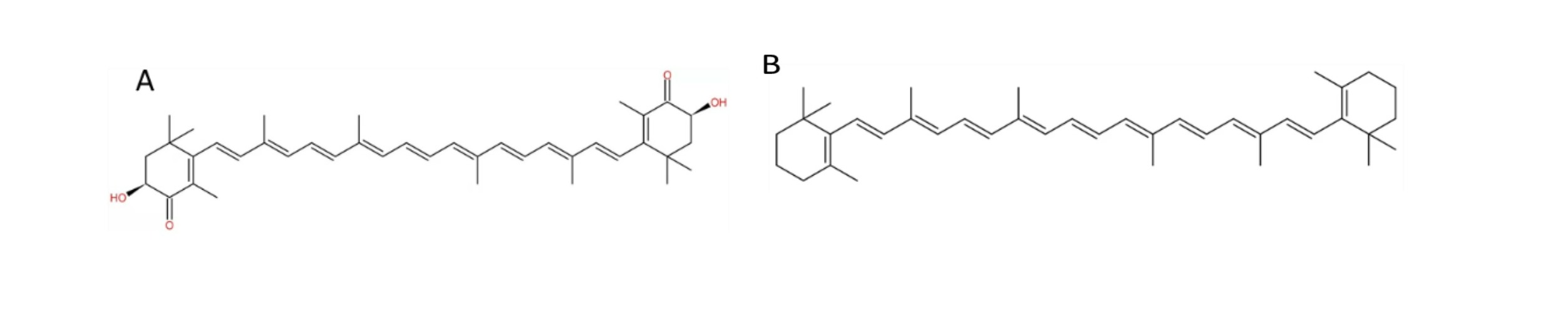
Figure 2. Chemical structure of ASX (A) and β-carotene (B).
2. Method
To conduct a comprehensive systematic literature search, the controlled vocabulary and free text terms and Boolean operators “AND” and “OR” were used. The following text words: ("astaxanthine”OR“astaxanthine”OR“astaxanthin”OR “astaxanthins” OR“ASX” OR “carotenoid”) AND (“skin” OR “skin derm *”) appeared in all fields of the articles. Multiple databases, including PubMed, Scopus, and Web of Science, were searched, yielding articles published in English between 2001 and 2021.
A study was included if it met all of the inclusion criteria, and a study was excluded if it met one or more of the exclusion criteria.
The following data were extracted: study design, study populations (age (years), gender, country and health conditions), the number of individuals in each group (intervention or control), study duration (weeks), content and dosage (mg/d) of each group, a form of applications, conditions of test rooms (°C room temperature (R.T.); % relative humidity (R.H.), seasons during study periods, UV-applications, measurement instruments, and skin parameters (measuring sites) with units were transcribed into a table.Other captured data, including the baseline and endpoint measure (mean value) and its variability (either standard deviation or standard error of the mean), were extracted by applying GetData Graph Digitizer as most publications presented results into figures.
The randomised, controlled studies (RCTs) were grouped according to the similarity of each outcome related to skin ageing, and meta-analysis was conducted for each group independently using Review Manager 5.4. And the statistical analysis used the random-effect model to calculate the mean difference and standard deviations (SDs) for continuous variables. Although the included RCTs have assessed several skin ageing parameters, meta-analysis was conducted only if there were at least 3 available identified studies for each parameter.
Some of the RCTs measured skin elasticity according to gross elasticity (R2), net elasticity (R5), biological elasticity (R5) and analysed wrinkles based on several parameters: deepest point of the deepest wrinkle, mean depth of the deepest wrinkle, the maximum width of the deepest wrinkle, the area ratio of all wrinkles, mean depth of all wrinkles and volume ratio of all wrinkles. This meta-analysis extracted and analysed values of gross elasticity (R2) and mean depth of all wrinkles because they suggested the whole changes in skin elasticity and wrinkles during study periods better than other parameters.
As Higgins I (I^2) represents the level of heterogeneity among the included studies and measures the proportion of inconsistency that cannot be explained by chance alone [19], ranging from 0% to 100%. I^2 < 25% indicated very low, 25% < I^2 < 50% reported low heterogeneity, 50% < I^2 < 75% indicated moderate heterogeneity, whereas I^2 ≥ 75% corresponded to substantial-high heterogeneity [20]. If there was a small or absent heterogeneity, a fixed-effects model was used in the meta-analysis [20]. As included studies did not apply the same outcome measure and scales, the standardized mean difference was proposed to be used [20].
The probability (p) value of ≤0.05 was regarded as statistically significant. The result of the overall effect test was shown as a standardized mean difference and 95% confidence interval (CI). The small effect sizes were 0.2 and below, the medium size ranged from 0.3 to 0.7 and the large effect size were 0.8 and above.
The methodological quality of included studies was systematically assessed using Cochrane Collaboration's tool for evaluating the risk of bias (RoB) [21], which involves seven items, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Following the guidelines in the Cochrane handbook, there are three options for judgments: low risk of bias in green, unclear risk of bias in yellow, and high risk of bias in red, respectively [22].
3. Results
The literature search finally identified 11 full-text publications.The eight RCTs involved 162 subjects in the ASX supplement group and 131 subjects in the placebo group. They were initially divided into three groups according to the measured outcomes, including moisture content, skin elasticity and wrinkle depth and then subjected to the meta-analysis.
Figure 4A displays the forest plot of the meta-analysis of five studies with respect to combined moisture content estimates comparing the placebo group with the group of participants orally supplemented with ASX. For this outcome, supplementation resulted in statistically significant improvement (Z = 2.17, p = 0.03), as based on the overall effect size of 0.53 (95% CI = 0.05, 1.01).
In the meta-analysis with 6 trials (Figure 4B) that evaluated skin elasticity, oral ASX supplementation also significantly improved the outcome (Z = 2.61, p = 0.009) in comparison to the placebo group, with an overall effect size of 0.77 (95% CI = 0.19, 1.35).
No significant differences (Z = 1.58, p = 0.11) were identified in studies between the ASX treatment and placebo groups in studies evaluating the wrinkle depth. Figure 4C shows a general effect size of −0.26 (95% CI = −0.58, 0.06).
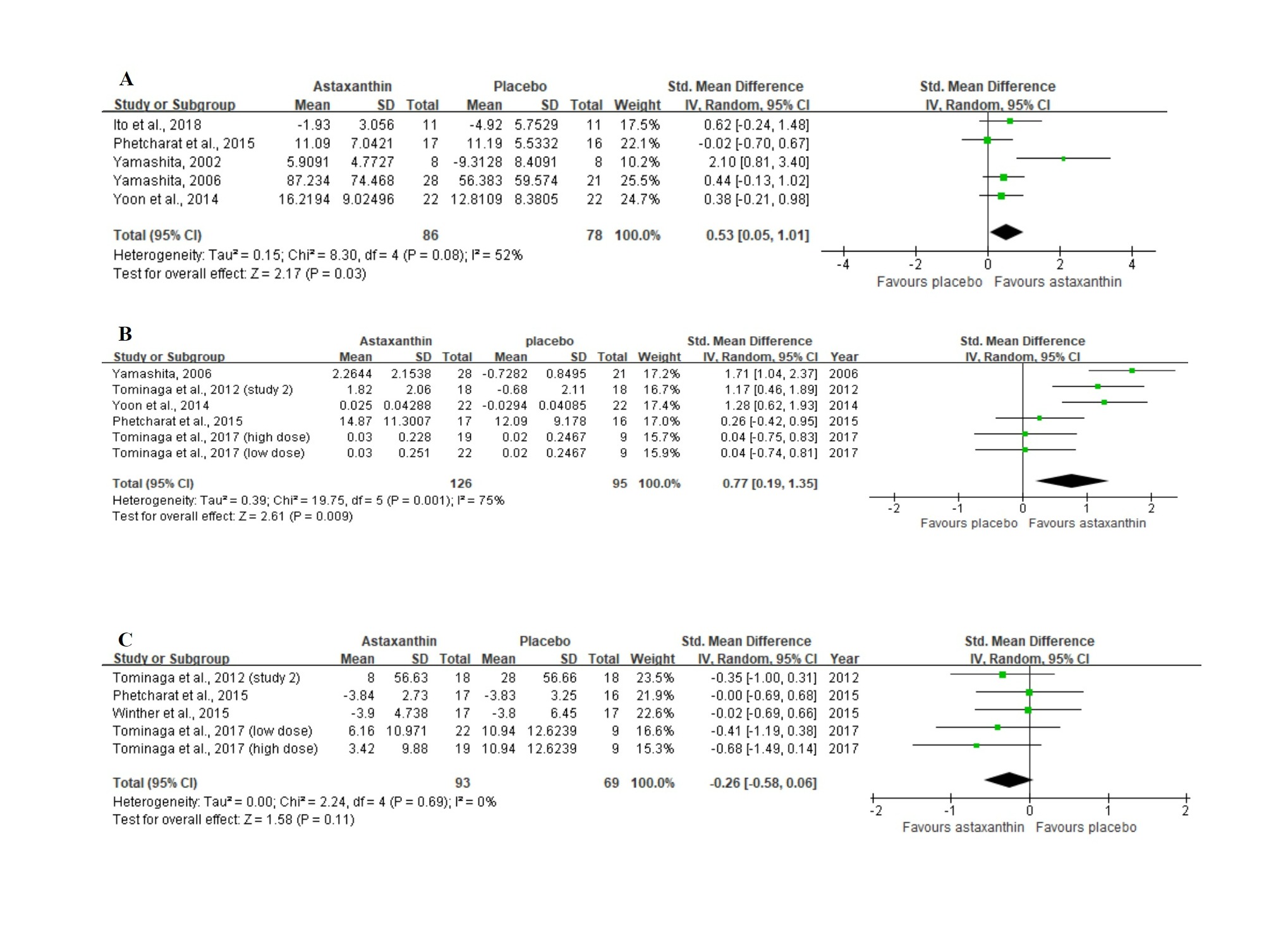 Figure 4. Forest plots summarizing the impact of ASX oral supplementation on (A) the increase in moisture content (µS), and (B) the increase in skin elasticity (%) using a random-effects model, as well as (C) the reduction in wrinkle depth (µm) using a fixed-effects model. Pooled summary data are presented as mean differences compared to control. The area of each green symbol is proportional to the weight of the study. Squares represent the standard mean differences, bars represent the 95% CI, and diamonds show the pooled effect.
Figure 4. Forest plots summarizing the impact of ASX oral supplementation on (A) the increase in moisture content (µS), and (B) the increase in skin elasticity (%) using a random-effects model, as well as (C) the reduction in wrinkle depth (µm) using a fixed-effects model. Pooled summary data are presented as mean differences compared to control. The area of each green symbol is proportional to the weight of the study. Squares represent the standard mean differences, bars represent the 95% CI, and diamonds show the pooled effect.
TEWL was reduced from baseline by 49.52% after administrating with 2 mg/d of ASX and 3 mg/d of collagen hydrolysate for 12 weeks while there was a 36.96% of TEWL reduction in the control group [9]. It is consistent with another study that six weeks of 6 mg/d of ASX supplementation resulted in a significant decrease in TEWL from the baseline while there was an increase in TEWL in the control group (study 2) [23].
Sebum content of the cheek has been maintained by four weeks of oral ASX and tocotrienol intake from baseline, while sebum content was decreased by 7.21 (without unit) in the placebo group at the end of the study [24]. A substantial reduction in sebum oil of cheek from baseline was observed after six weeks of applications of 6 mg/d of ASX oral supplementation compared to the placebo group [23].
Two open-label prospective studies measured the effects of topical and oral-topical ASX applications on skin ageing
The moisture content of the left outer canthus was significantly increased from baseline by 3.32% after three week-topical administration of 280 mg/d of ASX cream (study 1) [25]. The improvements in skin conditions such as dryness, flushing, itching and inconsistency with makeup were observed from questionnaire responses after three weeks of repeated ASX topical applications (study 1) [25]. The second study by Seki et al. (2001) [25] also showed a 106.67% elevation in skin moisture content of left outer canthus after two-week application of 140 mg/d of ASX-based cream in participants with dried and mixed skin. An antiwrinkle effect of ASX under eyes and outer canthus was observed in three subjects with different skin types by using magnified photographs. Skin conditions including moistness, smoothness, and elasticity obtained from skin-palpation and skin inspection by beauty specialists, were improved in all three subjects.
Significant wrinkle reductions and elasticity improvements were observed at outer canthus by 2.27% and 3.39%, respectively, after eight weeks of applications with 6 mg/d of oral ASX capsules and 94.18 mg/d of topical ASX cream [23]. A combination of ASX techniques showed a significant increase in moisture content of the corneocyte layer, the total area of the corneocyte, and the mean depth of skin texture in the cheek in 10 participants with dried skin [23].
Figure 5 shows the assessment of RoB at the domain level revealed an unclear RoB for most studies.
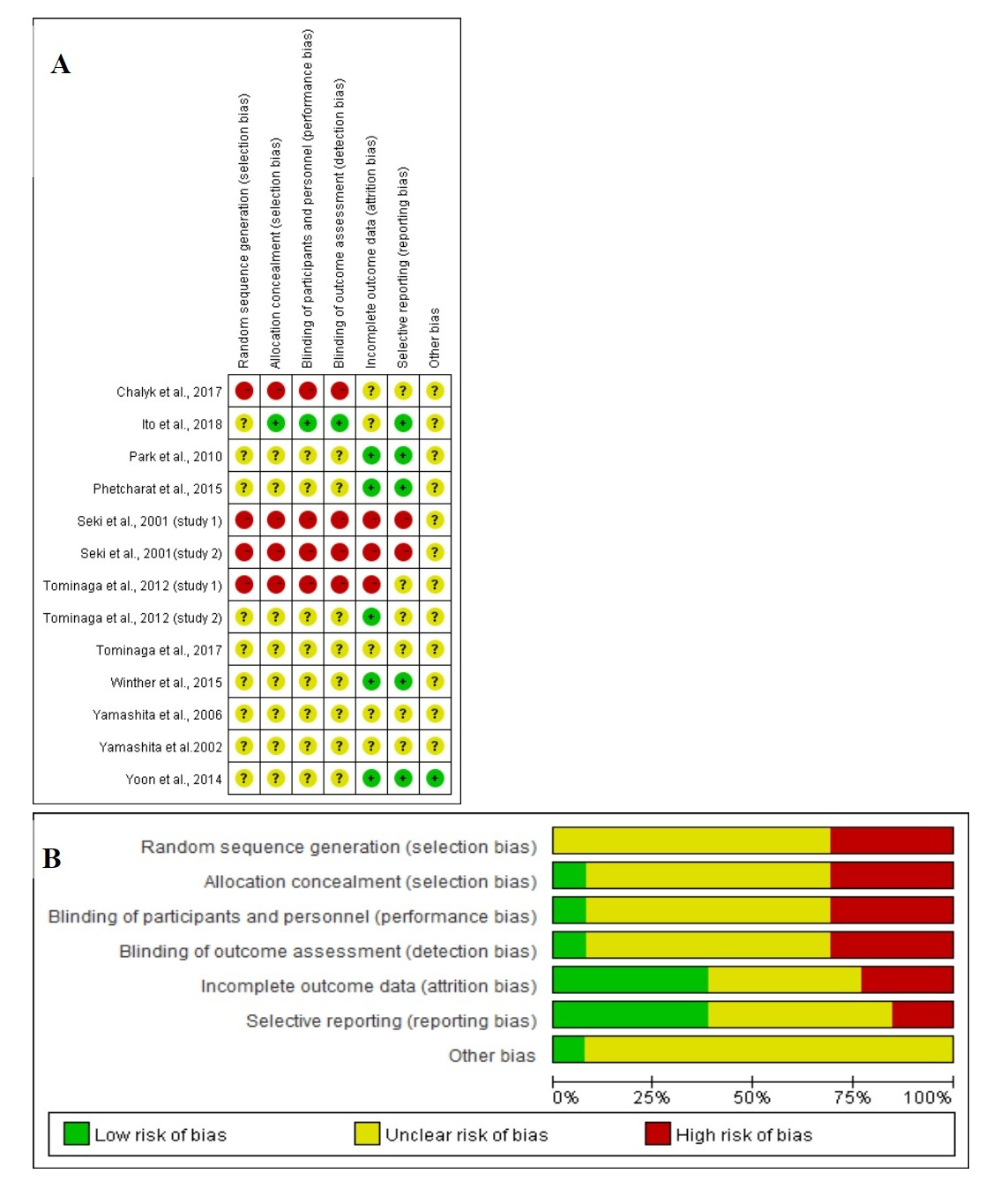
Figure 5. (A) Graph of RoB for each study according to the seven domains defined by Cochrane Collation’s tool. (B) Review of each RoB item presented as percentages across all included studies according to the judgments of the author.
4. Discussion
Given the results of the meta-analysis and weak evidence from open-label, prospective studies, ASX oral and/or topical applications may delay and improve the signs of skin ageing by enhancing moisture content and skin elasticity, reducing facial wrinkles and sebum oil due to its antioxidant, anti-inflammatory effects and improved effects on skin barrier integrity.
这些发现仅与之前的叙述性评论部分一致,表明 ASX 口服应用可能阻止了健康中年参与者的皮肤老化,这些参与者在基线时具有明确的年龄相关体征[ 2 ] [ 14 ] [ 26 ]。
由于证据的可靠性和强度受到样本量小、研究设计不完善、数据提取方法和潜在利益冲突的限制。未来的研究有必要设计更大规模的随机、盲法、对照试验,在不同地区招募更多的中年健康女性和男性参与者,尤其是西方国家的人,使用更客观的皮肤病学评估和报告原始数据。还需要应用超临界 CO2 萃取技术来生成无溶剂和分离形式的 ASX。
This entry is adapted from the peer-reviewed paper 10.3390/nu13092917
References
- Kuhn, R. The coloring matters of the lobster (Astacus gammarus L.). Z. Angew. Chem. 1938, 51, 465–466.
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2021, 18, 169–182.
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364.
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571.
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178.
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208.
- Natsuga, K. Epidermal barriers. Cold Spring Harb. Perspect. Med. 2014, 4, a018218.
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and-12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816.
- Gilchrest, B.; Yaar, M. Ageing and photoageing of the skin: Observations at the cellular and molecular level. Br. J. Dermatol. 1992, 127, 25–30.
- Tyrrell, R.M. Activation of mammalian gene expression by the UV component of sunlight—From models to reality. Bioessays 1996, 18, 139–148.
- Kuwazuru, O.; Miyamoto, K.; Yoshikawa, N.; Imayama, S. Skin wrinkling morphology changes suddenly in the early 30s. Ski. Res. Technol. 2012, 18, 495–503.
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 17–35.
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522.
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38.
- Yoshihisa, Y.; Andoh, T.; Matsunaga, K.; Rehman, M.U.; Maoka, T.; Shimizu, T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS ONE 2016, 11, e0152288.
- Patruno, C.; Napolitano, M.; Argenziano, G.; Peris, K.; Ortoncelli, M.; Girolomoni, G.; Offidani, A.; Ferrucci, S.; Amoruso, G.; Rossi, M. Dupilumab therapy of atopic dermatitis of the elderly: A multicentre, real-life study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 958–964.
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385.
- Sandercock, P. The Authors Say: ‘The Data Are Not So Robust because of Heterogeneity’—So, How Should I Deal with This Systematic Review? Cerebrovasc. Dis. 2011, 31, 615–620.
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid.-Based Healthc. 2015, 13, 196–207.
- Higgins, J.P.; Green, S.E. (Eds.) The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4.
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928.
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 2168.
- Yamashita, E. Cosmetic benefit of dietary supplements including astaxanthin and tocotrienol on human skin. 2002. Food Style 2002, 21, 6.
- Seki, T.; Sueki, H.; Kono, H.; Suganuma, K.; Yamashita, E. Effects of astaxanthin from Haematococcus pluvialis on human skin-patch test; skin repeated application test; effect on wrinkle reduction. Fragr. J. 2001, 12, 98–103.
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27.
