Acting as the primary link between mother and fetus, the placenta is involved in regulating nutrient, oxygen, and waste exchange; thus, healthy placental development is crucial for a successful pregnancy. In line with the increasing demands of the fetus, the placenta evolves throughout pregnancy, making it a particularly difficult organ to study. Research into placental development and dysfunction poses a unique scientific challenge due to ethical constraints and the differences in morphology and function that exist between species. An alternative is to create an in vitro model of the human placenta. Advancements in the differentiation of human induced pluripotent stem cells (hiPSCs), microfluidics, and bioprinting have each contributed to the development of new models, which can be designed to closely match physiological in vivo conditions. By including relevant placental cell types and control over the microenvironment, these in vitro models can reveal clues to the pathogenesis of placental dysfunction and facilitate drug testing across the maternal-fetal interface.
- placenta
- maternal-fetal interface
- trophoblast invasion
- bioprinting
- microfluidics
- placenta-on-a-chip
- in vitro models
1. Introduction
The human placenta is a crucial organ that supports fetal development throughout gestation. Placental growth and function are precisely regulated to ensure effective circulation of oxygen and nutrients, removal of waste, generation and release of metabolites, and protection against diseases, infections, and xenobiotic transfer to the fetus[1]. Considering its vital role, it is essential to understand placental development and the causes of its dysfunction. However, due to ethical concerns, our understanding of the placenta is largely derived from explants at term or from unsuccessful pregnancies. Explants have provided many clues into pathological pregnancies, such as fetal growth restriction, pre-eclampsia, and stillbirth at varied stages of disease[2][3][4]. However, explants begin to degenerate within hours after collection, making experimentation with human tissue challenging. Efforts have been made to develop accurate animal models[5]; however, considerable differences between species make it difficult to develop a non-primate animal model that fully mimics human placentation[6][7]. Rodent models are useful for understanding specific aspects of placentation, but many processes are difficult to assess in vivo. Ultimately, bioengineered in vitro models promise to bridge the gap between species and offer precise control over the microenvironment to recapitulate specific aspects of human placentation in health and disease[8].
2. Development and Functions of the Human Placenta
The placenta, a fetal organ, forms shortly after fertilization and continues to change throughout pregnancy in response to the metabolic demands of the fetus. Placentation begins post-fertilization when the blastocyst attaches to the inner layer (endometrium) of the uterus. The blastocyst then begins to invade the endometrium with the help of its outer layer of cells, termed trophoblasts. This fetal trophoblastic layer is divided into two cell types, an external multinucleated syncytiotrophoblast layer (the invasive trophoblasts) and an inner cytotrophoblast layer. About two weeks after fertilization, the external syncytiotrophoblast forms preliminary fluid-filled villi structures directed outward, towards the decidual layer of the mother’s uterus (Figure 1a). Then, the cytotrophoblasts proliferate and migrate through the syncytiotrophoblastic layer to form the primary villi[9]. Soon, these villi expand and become vascularized with fetal placental vessels. Meanwhile, trophoblasts remodel the maternal spiral arteries of the decidua, which become dilated, allowing for maternal blood to fill the intervillous space. As a result, there is a large surface area for the exchange of nutrients traveling from the mother’s circulation into the intervillous spaces, through the trophoblast layers, and into the closed placental circulation of the villi, which nourishes the fetus via the umbilical cord[10][11]. By the second trimester, the main features of the mature placenta are formed (Figure 1b).
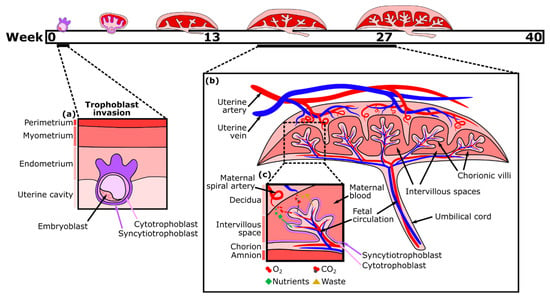
Nutrient exchange between maternal and fetal blood is facilitated largely by the syncytiotrophoblast, which is one continuous multinucleated layer of cells ( Figure 1c). The ability of nutrients to cross this layer depends on transporter proteins and its thickness, which is reduced near the vascularized parts of the villi[12]. Small hydrophobic molecules, such as oxygen and carbon dioxide, can easily diffuse across plasma membranes in response to differences in the concentration gradient between maternal and fetal blood, which varies with the maternal blood supply, environment, and rate of blood flow. Partial pressure of oxygen in the maternal blood is considerably higher than in the fetal blood, while carbon dioxide is more abundant in the fetal blood. Oxygen exchange is also facilitated by fetal hemoglobin having a higher affinity for oxygen than that of an adult[13]. Consequently, oxygen diffuses through the placenta from the maternal to the fetal blood, while carbon dioxide diffuses in the opposite direction. Transport of large (molecular weight > 1 kDa) and hydrophilic molecules, however, is size restricted and diffusion limited, therefore various transporter proteins are necessary to increase flux[14][15].
The placenta also functions as an immunological barrier, countering the maternal immune response that would normally cause rejection of the fetus, finally leading to spontaneous abortion[16]. Still, maternal antibodies (immunoglobulin G (IgG)) are actively transported across the placenta by neonatal Fc receptors (FcRns), conferring protection against infections to the fetus and the neonate during the first months of life [17].
In addition to its barrier function, the placenta acts as an endocrine organ. For example, to prevent the progression of the menstrual cycle and the loss of the endometrial lining, the syncytiotrophoblast releases human chorionic gonadotropin (HCG). HCG prolongs the life of the corpus luteum, which is thus able to continue releasing progesterone and promote the healthy function of endometrial vasculature, preventing its deterioration and loss[18]. The placenta also produces placental growth hormone (PGH), which is structurally very similar to pituitary growth hormone and eventually completely replaces it[19]. Importantly, the placenta supports pregnancy and fetal growth by selectively secreting the steroids estrogen and progesterone[19].
3. In Vitro Models of the Placental Barrier
A variety of in vitro models have been developed to study different aspects of placental biology; however, one aspect of particular focus is the maternal-fetal interface as a barrier (Figure 2a). Cells derived from gestational choriocarcinoma[20][21] or immortalized trophoblasts[22] have been widely used to represent both villous and extravillous trophoblasts as cultured monolayers. The monolayers have been grown on plates or semipermeable membranes (transwell inserts) and have been employed to investigate hormone secretion, transcellular transport of glucose, environmental toxicants, and susceptibility to parasite infection[23][24][25]. Despite their simplicity, cell monolayer models can be an effective first step to studying the mechanisms and properties of the human placental barrier. However, by focusing on just one aspect of the placenta (trophoblasts), these models lack physiological complexity and a comparable cellular microenvironment. Kreuder et al. addressed some of these drawbacks by including essential components of the placental villi, such as fibroblasts and endothelial cells[26], as represented in Figure 2b. Their model involved bioprinting a methacrylated gelatin membrane (GelMA), which mimics extracellular matrix (ECM) features, containing primary placental fibroblasts, to simulate villous stroma. BeWo trophoblasts and primary human placental endothelial cells were cultured on either side of this printed membrane, thus representing a more complex model of human placental villi[26]. Barrier properties were assessed by two permeability assays: one using a fluorescently labeled molecule to measure solute flux, and the other by impedance-based measurements using a transepithelial electrical resistance (TEER) system. Their results showed that the bioprinted membrane presents physiological ECM-like features, such as a lower elasticity, which resembles that of placental tissue, in comparison with filter membrane-based systems. Moreover, TEER values were higher when BeWo trophoblasts were cultured on the membrane containing fibroblasts rather than in monotypic cultures, demonstrating a reduction in permeability (reduced leakiness) due to the incorporation of the stromal compartment.
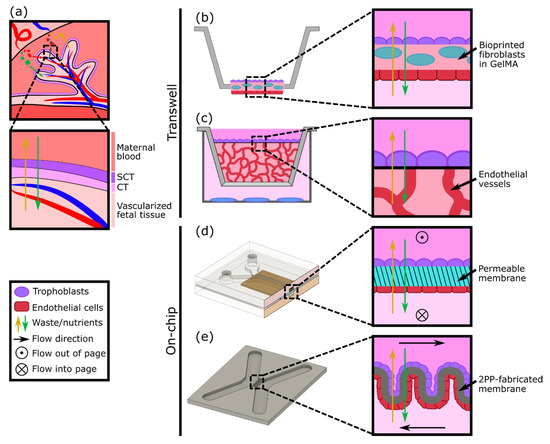
In vitro models of the placental barrier have also recently incorporated 3D vascularized networks. For example, Nishiguchi et al. used a modified transwell system to seed a layer of laminin and collagen-coated trophoblasts (either BeWo or primary cytotrophoblasts) onto a thick layer of self-assembled capillary networks, formed from primary fibroblasts (normal human dermal fibroblasts (NHDFs)) and human umbilical vein endothelial cells (HUVECs) in a fibrin hydrogel[27] (Figure 2c). This model was employed to examine cell damage signaling across the barrier by exposing rat embryonic cortical neurons to conditioned medium collected from the in vitro placental barriers assembled with direct or indirect contact with the vascular bed. Although the vessels were not perfused, the presence of the vasculature resulted in a reduction in neuron dendrite length, providing evidence of crosstalk between the trophoblasts and the endothelium.
Considering the multilayered structure and physiologic microenvironment critical to placental barrier function, other groups have also attempted to generate more complex models, including both an endothelial (representative of the fetal vessels) and trophoblast component. For instance, cocultures of endothelial and trophoblast cells have been combined in sandwiched monolayers on chip, which allow for exposure to fluid flow mimicking the hemodynamic shear stress present in maternal and fetal compartments (Figure 2d). Blundell et al. generated a two-layer polydimethylsiloxane (PDMS) device with two channels separated by a thin porous membrane, which allows for constant perfusion with culture media[28]. BeWo trophoblasts were cultured on the upper side of the membrane and human placental vascular endothelial cells (HPVECs) on the lower side. This coculture model recapitulated structural features of the maternal-fetal interface and showed the expression and physiological localization of placental transporter proteins. The authors observed more complete formation of dense microvilli projections on the apical surface of the trophoblast cells when cultured under fluid shear stress conditions, when compared with static culture. Moreover, they found that inclusion of the fetal endothelium was crucial to replicate physiological maternal-fetal glucose transport, as confirmed by comparing with the glucose transfer rates measured across two other types of barriers: a cell-free barrier and a trophoblast monolayer without endothelium[28]. In a similar approach, Lee et al. cultured JEG-3 trophoblasts and HUVECs on either side of a solidified collagen membrane and subjected each side to dynamic flow conditions. The system facilitated cell proliferation and the formation of confluent monolayers into a placental barrier model, which demonstrated different glucose transport rates depending on the presence of the epithelium and in accordance with findings from Blundell’s model[17]. More recently, a similar microfluidic two-channel design with a polyethylene (PETE) membrane separating monolayers of BeWo trophoblasts and HUVECs was tested to examine caffeine transport across the placenta, a molecule that cannot be fully metabolized by a developing fetus[29]. This study provided new insights into the extent of caffeine transfer from mother to fetus and demonstrated the utility of the system for future xenobiotic compound testing.
Another way to achieve the complex geometry of the placental villous membrane, while bypassing the use of flat cell monolayers, was proposed by Mandt et al., who developed a barrier model using a high-resolution three-dimensional (3D) printing method called two-photon polymerization (2PP)[30]. A villi-like convoluted surface within a microfluidic device with two separate channels was shaped by 2PP from a modified gelatin-based hydrogel material (GelMA), mimicking the basal membrane of the placenta (Figure 2e). To mimic the fetal and maternal compartments, HUVEC and BeWo trophoblasts were then seeded on either side of the membrane and cultured under constant flow. The authors studied transcellular transport across this barrier and demonstrated in vivo-like properties by showing the permeability of sugar-sized molecules (riboflavin, 350 Da) and the impermeability of larger ones (dextran, 200 kDa).
4. In Vitro Models of Trophoblast Invasion
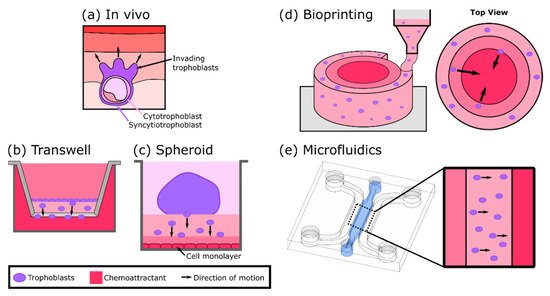
5. Three-Dimensional Models to Study Placental Dysfunction, Infections, and Maternal-Fetal Toxicology
Besides the defective remodeling of the spiral arteries, many other aspects of placental dysfunction are associated with altered placental development and the onset of pregnancy complications. These events, which include the impairment of villous tree maturation[43], the detrimental effects of pathogen infection [44], and the response to drugs and environmental cues[45][46], still need further investigation. As discussed previously, the advent of technologies such as bioprinting and microfluidic-based organs-on-a-chip have facilitated the recapitulation of critical placental functions and stages of development, raising the possibility to apply these models to study the mechanisms underlying these aberrant events. For example, a work from Haase et al. brought new insight into placental vasculopathy, showing that pericytes (mural cells of the microvasculature) contribute to growth restriction of fetal microvessels grown in microfluidic devices[47]. Moreover, the results showed PE-like effects, including upregulation of inflammatory cytokines, hyperproliferation of stromal cells, dysfunctional barrier properties, and immune cell infiltration.
Microfluidic technologies have also been implemented to explore the impact of pathogenic infections during pregnancy. Zhu et al. generated a multilayered microfluidic placental barrier-on-a-chip model to investigate the placental inflammatory responses to bacterial infection[48]. When Escherichia coli was applied to the maternal side of the chip, trophoblast cells triggered an acute inflammatory response by secreting interleukin-1α, IL-1β, and IL-8 cytokines, followed by the adhesion of maternal macrophages. More recently, a microfluidic organ-on-a-chip model comprising the decidua and the fetal chorionic and amnionic membranes was generated to track the propagation of infection and inflammation across the maternal-fetal interface[49]. This four-chamber system containing primary cells from the maternal-fetal interface and a collagen matrix mimics cellular features seen in the native tissue, such as morphology, cellular transitions, migration, and production of nascent collagen. The ascending infection and consequent inflammation were tracked by examining the propagation of lipopolysaccharide (LPS) from the decidua to the amnion. The results demonstrated the disruption of the maternal-fetal interface integrity during ascending infection due to an imbalanced immune response, an event that is associated with preterm birth[50].
This entry is adapted from the peer-reviewed paper 10.3390/mi12080884
References
- Maltepe, E.; Fisher, S.J.; Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552, https://doi.org/10.1146/annurev-cellbio-100814-125620.
- Higgins, L.E.; De Castro, N.R.; Addo, N.; Wareing, M.; Greenwood, S.; Jones, R.L.; Sibley, C.P.; Johnstone, E.; Heazell, A.; Placental Features of Late-Onset Adverse Pregnancy Outcome. PLoS ONE 2015, 10, e0129117, doi: 10.1371/journal.pone.0129117.
- Visiedo, F.; Bugatto, F.; Prado, R.Q.; Cózar-Castellano, I.; Bartha, J.L.; Perdomo, G.; Glucose and Fatty Acid Metabolism in Placental Explants from Pregnancies Complicated With Gestational Diabetes Mellitus. Reprod. Sci. 2015, 22, 798–801, https://doi.org/10.1177/1933719114561558.
- Gonzales, S.K.; Badell, M.; Cottrell, H.; Rimawi, B.; Deepak, V.; Sidell, N.; Rajakumar, A.; Villous explants from preeclamptic placentas induce sFlt1 in PBMCs: An ex vivo co-culture study. Pregnancy Hypertens. 2018, 12, 40-46, doi: 10.1016/j.preghy.2018.02.006.
- Grigsby, P.L.; Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 011-016, doi: 10.1055/s-0035-1570031.
- Schmidt, A.; Prieto, D.M.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R.; Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65-71, doi: 10.1016/j.jri.2015.03.001.
- Walker, N.; Filis, P.; Soffientini, U.; Bellingham, M.; O’Shaughnessy, P.J.; Fowler, P.A.; Placental transporter localization and expression in the Human: The importance of species, sex, and gestational age differences. Biol. Reprod. 2017, 96, 733-742, https://doi.org/10.1093/biolre/iox012.
- Wheeler, M.L.; Oyen, M.L.; Bioengineering Approaches for Placental Research. Ann. Biomed. Eng. 2021, 49, 1805–1818, https://doi.org/10.1007/s10439-020-02714-7.
- Turco, M.Y.; Moffett, A.; Development of the human placenta . Development 2019, 146, dev163428, https://doi.org/10.1242/dev.163428.
- Huppertz, B.; Weiss, G.; Moser, G.; Trophoblast invasion and oxygenation of the placenta: Measurements versus presumptions. J. Reprod. Immunol. 2014, 101-102, 74-79, https://doi.org/10.1016/j.jri.2013.04.003.
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J.; Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496, https://doi.org/10.1007/s00018-019-03104-6.
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S.; The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71, https://doi.org/10.1007/s11095-017-2286-0.
- Manning, J.M.; Manning, L.R.; Dumoulin, A.; Padovan, J.C.; Chait, B.; Embryonic and Fetal Human Hemoglobins: Structures, Oxygen Binding, and Physiological Roles. Prokaryotic Cytoskelet. 2020, 94, 275-296, DOI: 10.1007/978-3-030-41769-7_11.
- Burton, G.J.; Fowden, A.L.; The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066, doi: 10.1098/rstb.2014.0066.
- Gupta, R.K.; Gupta, R.C.; Chapter 68—Placental Toxicity. Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA 2017, pp., 1301–1325, https://doi.org/10.1016/B978-0-12-804239-7.00068-8.
- Leber, A.; Zenclussen, M.L.; Teles, A.; Brachwitz, N.; Casalis, P.; El-Mousleh, T.; Jensen, F.; Woidacki, K.; Zenclussen, A.; Pregnancy: Tolerance and Suppression of Immune Responses . In Suppression and Regulation of Immune Responses: Methods and Protocols; Cuturi, M.C., Anegon, I., Eds.; Humana Press: Totowa, NJ, USA 2011, pp., 397–417, DOI: 10.1007/978-1-60761-869-0_25.
- Simister, N.E.; Placental transport of immunoglobulin G . Vaccine 2003, 21, 3365–3369, https://doi.org/10.1016/S0264-410X(03)00334-7.
- Srisuparp, S.; Strakova, Z.; Fazleabas, A.T.; The Role of Chorionic Gonadotropin (CG) in Blastocyst Implantation . Arch. Med. Res. 2001, 32, 627–634, https://doi.org/10.1016/S0188-4409(01)00330-7.
- Evain-Brion, D.; Malassine, A.; Human placenta as an endocrine organ . Growth Horm. IGF Res. 2003, 13, S34-S37, https://doi.org/10.1016/S1096-6374(03)00053-4.
- Pattillo, R.A.; Gey, G.O.; The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 28, 1231–1236, DOI: Published July 1968.
- Kohler, P.O.; Bridson, W.E.; Isolation of Hormone-Producing Clonal Lines of Human Choriocarcinoma. J. Clin. Endocrinol. Metab. 1971, 32, 683-687, https://doi.org/10.1210/jcem-32-5-683.
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K.; Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan . Exp. Cell Res. 1993, 206, 204-211, https://doi.org/10.1006/excr.1993.1139.
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P.; A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 5892, https://doi.org/10.1038/s41598-017-06364-z.
- Almeida, M.P.O.; Ferro, E.A.V.; Briceño, M.P.; Oliveira, M.C.; Barbosa, B.F.; Silva, N.M.; Susceptibility of human villous (BeWo) and extravillous (HTR-8/SVneo) trophoblast cells to Toxoplasma gondii infection is modulated by intracellular iron availability. Parasitol. Res. 2019, 118, 1559-1572, https://doi.org/10.1007/s00436-019-06257-2.
- Widhalm, R.; Ellinger, I.; Granitzer, S.; Forsthuber, M.; Bajtela, R.; Gelles, K.; Hartig, P.-Y.; Hengstschläger, M.; Zeisler, H.; Salzer, H.; et al. Human placental cell line HTR-8/SVneo accumulates cadmium by divalent metal transporters DMT1 and ZIP14. Metallomics 2020, 12, 1822-1833, https://doi.org/10.1039/d0mt00199f.
- Kreuder, A.-E.; Bolaños-Rosales, A.; Palmer, C.; Thomas, A.; Geiger, M.-A.; Lam, T.; Amler, A.-K.; Markert, U.R.; Lauster, R.; Kloke, L.; et al. Inspired by the human placenta: A novel 3D bioprinted membrane system to create barrier models. Sci. Rep. 2020, 10, 15606, https://doi.org/10.1038/s41598-020-72559-6.
- Nishiguchi, A.; Gilmore, C.; Sood, A.; Matsusaki, M.; Collett, G.; Tannetta, D.; Sargent, I.L.; McGarvey, J.; Halemani, N.D.; Hanley, J.; et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials 2019, 192, 140-148, https://doi.org/10.1016/j.biomaterials.2018.08.025.
- Blundell, C.; Tess, E.R.; Schanzer, A.S.R.; Coutifaris, C.; Su, E.J.; Parry, S.; Huh, D.; A microphysiological model of the human placental barrier. Lab Chip 2016, 16, 3065–3073, https://doi.org/10.1039/C6LC00259E.
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N.; Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob. Chall. 2019, 3, 1800112, https://doi.org/10.1002/gch2.201800112.
- Mandt, D.; Gruber, P.; Markovic, M.; Tromayer, M.; Rothbauer, M.; Kratz, S.R.A.; Ali, F.; Van Hoorick, J.; Holnthoner, W.; Mühleder, S.; et al. Fabrication of placental barrier structures within a microfluidic device utilizing two-photon polymerization. Int. J. Bioprint. 2018, 4, 144, http://dx.doi.org/10.18063/ijb.v4i2.144.
- Huppertz, B.; Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2019, 21, 289, https://doi.org/10.3390/ijms21010289.
- Lacroix, M.-C.; Guibourdenche, J.; Fournier, T.; Laurendeau, I.; Igout, A.; Goffin, V.; Pantel, J.; Tsatsaris, V.; Evain-Brion, D.; Stimulation of Human Trophoblast Invasion by Placental Growth Hormone . Endocrinology 2005, 146, 2434-2444, https://doi.org/10.1210/en.2004-1550.
- Desforges, M.; Harris, L.K.; Aplin, J.D.; Elastin-derived peptides stimulate trophoblast migration and invasion: A positive feedback loop to enhance spiral artery remodelling. Mol. Hum. Reprod. 2015, 21, 95-104, https://doi.org/10.1093/molehr/gau089.
- Zhang, Z.; Li, P.; Wang, Y.; Yan, H.; Hypoxia-induced expression of CXCR4 favors trophoblast cell migration and invasion via the activation of HIF-1α. Int. J. Mol. Med. 2018, 42, 1508-1516, https://doi.org/10.3892/ijmm.2018.3701.
- Bojić-Trbojević, Ž.; Krivokuća, M.J.; Vilotić, A.; Kolundžić, N.; Stefanoska, I.; Zetterberg, F.; Nilsson, U.J.; Leffler, H.; Vićovac, L.; Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep. 2019, 9, 2136, https://doi.org/10.1038/s41598-018-38374-w.
- You, Y.; Stelzl, P.; Zhaing, Y.; Porter, J.; Liu, H.; Liao, A.; Aldo, P.B.; Mor, G.; Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am. J. Reprod. Immunol. 2019, 81, e13076, https://doi.org/10.1111/aji.13076.
- Kuo, C.-Y.; Guo, T.; Cabrera-Luque, J.; Arumugasaamy, N.; Bracaglia, L.; Garcia-Vivas, A.; Santoro, M.; Baker, H.; Fisher, J.; Kim, P.; et al. Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J. Biomed. Mater. Res. Part A 2018, 106, 1476-1487, https://doi.org/10.1002/jbm.a.36350.
- Ding, H.; Illsley, N.P.; Chang, R.C.; 3D Bioprinted GelMA Based Models for the Study of Trophoblast Cell Invasion. Sci. Rep. 2019, 9, 18854, https://doi.org/10.1038/s41598-019-55052-7.
- Kuo, C.-Y.; Eranki, A.; Placone, J.K.; Rhodes, K.R.; Aranda-Espinoza, H.; Fernandes, R.; Fisher, J.P.; Kim, P.C.W.; Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomater. Sci. Eng. 2016, 2, 1817-1826, https://doi.org/10.1021/acsbiomaterials.6b00031.
- Abbas, Y.; Oefner, C.M.; Polacheck, W.; Gardner, L.; Farrell, L.; Sharkey, A.; Kamm, R.; Moffett, A.; Oyen, M.L.; A microfluidics assay to study invasion of human placental trophoblast cells . J. R. Soc. Interface 2017, 14, 20170131, https://doi.org/10.1098/rsif.2017.0131.
- Pu, Y.; Gingrich, J.; Veiga-Lopez, A.; A 3-dimensional microfluidic platform for modeling human extravillous trophoblast invasion and toxicological screening . Lab Chip 2021, 21, 546-557, https://doi.org/10.1039/D0LC01013H.
- Armant, D.; Fritz, R.; Kilburn, B.; Kim, Y.; Nien, J.K.; Maihle, N.; Romero, R.; Leach, R.; Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta 2015, 36, 270-278, https://doi.org/10.1016/j.placenta.2014.12.006.
- Turowski, G.; Vogel, M.; Re-view and view on maturation disorders in the placenta. APMIS 2018, 126, 602-612, https://doi.org/10.1111/apm.12858.
- Weckman, A.M.; Ngai, M.; Wright, J.; McDonald, C.R.; Kain, K.C.; The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Front. Microbiol. 2019, 10, 1924, https://doi.org/10.3389/fmicb.2019.01924.
- Koren, G.; Ornoy, A.; The role of the placenta in drug transport and fetal drug exposure . Expert Rev. Clin. Pharmacol. 2018, 11, 373-385, https://doi.org/10.1080/17512433.2018.1425615.
- Mathiesen, L.; Buerki-Thurnherr, T.; Pastuschek, J.; Aengenheister, L.; Knudsen, L.E.; Fetal exposure to environmental chemicals; insights from placental perfusion studies. Placenta 2021, 106, 58-66, https://doi.org/10.1016/j.placenta.2021.01.025.
- Haase, K.; Gillrie, M.R.; Hajal, C.; Kamm, R.D.; Pericytes Contribute to Dysfunction in a Human 3D Model of Placental Microvasculature through VEGF-Ang-Tie2 Signaling. Adv. Sci. 2019, 6, 1900878, https://doi.org/10.1002/advs.201900878.
- Zhu, Y.; Yin, F.; Wang, H.; Wang, L.; Yuan, J.; Qin, J.; Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater. Sci. Eng. 2018, 4, 3356–3363, https://doi.org/10.1021/acsbiomaterials.8b00653.
- Richardson, L.S.; Kim, S.; Han, A.; Menon, R.; Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip 2020, 20, 4486-4501, https://doi.org/10.1039/D0LC00875C.
- Green, E.S.; Arck, P.C.; Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus . Semin. Immunopathol. 2020, 42, 413-429, https://doi.org/10.1007/s00281-020-00807-y.
- McBride, W.; Thalidomide and congenital abnormalities. Lancet 1961, 278, 1358, DOI:https://doi.org/10.1016/S0140-6736(61)90927-8.
- Blundell, C.; Yi, Y.-S.; Ma, L.; Tess, E.R.; Farrell, M.J.; Georgescu, A.; Aleksunes, L.; Huh, D.; Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier . Adv. Healthc. Mater. 2018, 7, 1700786, https://doi.org/10.1002/adhm.201700786.
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T.; Recent insights on indirect mechanisms in developmental toxicity of nanomaterials . Part. Fibre Toxicol. 2020, 17, 31, https://doi.org/10.1186/s12989-020-00359-x.
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J.; A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier . Toxicol. In Vitro 2019, 54, 105-113, https://doi.org/10.1016/j.tiv.2018.08.014.
