The redox active organic compounds and composites should meet the criteria of capacitive nature and have high chemical and electrochemical stability. Pseudocapacitive behavior that shows ideal characteristics as reflected in linear galvanostatic charge-discharge and rectangular voltammograms need to be targeted. Battery-like bulk faradaic behavior, which is not ideal for high-power (rate) capacitive applications but is often reported for supercapacitors, should be differentiated.
- organic redox active materials
- redox active polymers
- organic-carbon composite electrodes
- capacitive energy storage
- electrochemical capacitors
- capacitive electrodes
1. Introduction
Electrochemical capacitors (ECs) or supercapacitors, possessing high power densities and excellent cycle life, are one of the key enablers for clean and sustainable energy [1]. From the early conceptual development by Conway [2] in the last century to the latest advancement [3], capacitive materials have been differentiated from battery materials for their fast and reversible charge-discharge kinetics as well as long and stable cycles lives. These properties are originated from two main sources: electrical double-layer capacitance (EDLC) and pseudocapacitance [4][5]. High surface area carbon-based materials are mostly used for EDLC [2][6][7][8][9][10], which store charges through the electrostatic adsorption of ions from the electrolyte on the surface of the electrode. Pseudocapacitance stores and delivers charges through fast and reversible multiple electron transfer oxidation and reduction (redox) reactions [6][11], which can be 10 to 100 times greater than that of EDLC. While pseudocapacitive materials, including metal oxides [12][13], and conducting polymers [14][15][16], can provide higher specific capacitance and high energy densities, they also add significant cost from materials and processing.
Combining EDLC and pseudocapacitive materials in composite forms is a highly effective, viable and economical approach to leverage the best of both. This has stimulated significant research and many excellent reviews. Among those reports, the majority are based on carbon modified with metal oxides [1][12][13][17][18][19] as well as those well-known redox active conducting polymers (CPs) focusing on polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT) [20][21][22][23]. The inorganic-carbon composites have greatly improved the capacitance and long-term stability of the electrodes, but there are still challenges from environmental and sustainability standpoints, as metal oxides composites still require natural resources and extensive mining and extraction processes.
Although organic materials are abundant and often inexpensive to produce on a large scale, the common electrochemically active (or redox active) materials and composites have been limited to a few conducting polymers. There is a need to explore additional redox active organic compounds to increase the usage of these carbon-based materials and to complement the current capacitive material landscape. Moreover, a strong understanding of the interactions between organic compounds and carbon substrates is important for further development of advanced composites for high performance ECs and other future high power energy storage.
2. Redox Active Compounds and Carbon Substrates
Redox active compounds can be further categorized to small molecules, macrocycles and conducting polymers (CPs). Among small molecules and macrocycles, some promising examples include pyrenes derivatives [24][25], meta, para and ortho (m, p, o)-phenylenediamines [47–49], quinones [50–52], as well as porphyrin and phthalocyanine macrocycles [26][27][28][29]. The advantages of these molecules are their chemical tunability and their nitrogen and oxygen redox functional groups that can undergo reversible charge transfer processes. For instance, quinone based molecules have a high theoretical capacity, fast electron transfer kinetics, and low-cost synthesis [30]. Their redox activities stem from the reversible conversion of the quinone groups to hydroxyl groups. However, the quinone groups become unstable in electrolytes with pH greater than 7 [31], indicating that the redox activities of organic compounds also are electrolyte dependent. This is a critical point since the electrolyte system plays an importance role in the performance of the final composite electrode.
The electrochemistry of phenylenediamine (PD), a small molecule with two primary amines attached to the benzene ring is greatly influenced by their different positions. When a second amine group is in the para- or ortho- position, a greater redox current has been observed, attributed to the different hybridization state of the molecules. When para-phenylenediamine (p-PD) and ortho-phenylenediamine (o-PD) are in acidic environment, both can undergo reversible redox processes between the benzoid diamine and quinoid diimine with four resonance structures. Meanwhile, meta-phenylenediamine (m-PD) does not form the quinoid diimine, resulting to a lesser faradaic contribution to the charge storage process [32].
Carbonaceous materials have been the choice of electrode materials for ECs due to their own double-layer properties, in particular carbon allotropes that include biomass activated carbon (AC), carbon nanotubes (CNTs), graphene and carbon nano-onions ( Figure 1 ) [33][34][35][36][37][38]. Their intrinsic high surface area, adjustable pore structure and size distribution, good electrical conductivity, and chemical stability make them excellent substrates to anchor various type of redox active compounds for high performance electrodes. Furthermore, functional groups on the carbon substrate can contribute towards surface wettability, better electronic activity, pseudocapacitance and enlarged operating potential window [39]. Among surface functional groups, oxygen and nitrogen functionalities are often involved and can be introduced through oxidation, doping or using heteroatom-containing precursors. Other functionalities including sulphur [40], phosphorus and boron, alter the electronic properties of the surface according to their sizes and electronegativities [40][41][42].
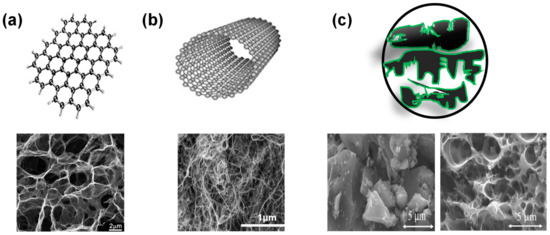
A wide variety of AC ( Figure 1c) have been used for commercial EDLC devices, partially due to their abundance and low-cost. ACs can be produced from different sources including coconut shell, wood, and other biomass waste through a 2-step process: thermal pyrolysis, and activation. This leads to a wide pore size distribution of micropores (˂2 nm), mesopores (2–50 nm), and macropores (˃50 nm) in AC structure. In addition to the low-cost, AC possess high chemical stability and high specific surface area up to 3000 m 2/g resulting in specific capacitance ranging from 70–200 F/g [47][43][48][49]. Developing sustainable approaches to produce high performance AC materials is critically important to today’s carbon neutrality and is still an ongoing challenge being researched extensively.
The main issue pertaining to carbon substrates is controlling surface features, such as the pore size, shape, and surface functionalities to enable bonding redox active species and promote a high utilization of their redox centre. For instance, leveraging the inherent porosity of waste biomass and improving the interlayer spacing of graphene sheets could be strategies to facilitate molecules and polymers onto the substrate surface [33][50]. Incorporating surface functionalities to the carbon substrate could aid in anchoring organic redox materials and further improve the charge storage properties.
3. State-of-the-Art of Redox Active Organic-Carbon Composites
3.1. Computational modelling
Most of the capacitive organic-carbon composites rely on noncovalent interactions between the electroactive layers and carbon substrate such as van der Waals (vdW) forces, polymer wrapping, hydrogen bonding, and electrostatic interactions [51][52]. The adsorption energies for noncovalent interaction have been quantified through DFT and can vary widely but is generally between ~10–251 kJ/mol (0.10–2.59 eV) ( Figure 2a) [53][54].
The reactive force field interatomic potentials (ReaxFF) approach within MD has enabled the classical treatment of reactive chemistries with considerations for bond orders [55][56]. Using MD with ReaxFF, Benda et al. demonstrated the distribution in adsorption energies for polyfluorene and fluorene/carbazole copolymers with various functional groups noncovalently binding to CNT [57]. The study showed the effects of π-π stacking, and steric repulsion effects between different polymer chains on the stability of the composite, as shown in Figure 2 g Adsorption energies per monomer in the range of 115.9 to 190.8 kJ/mol were calculated for a range of CNT diameters and polymer lengths. This approach can provide insights into the stability of promising redox active polymers on a carbon substrate prior to experimental investigations.
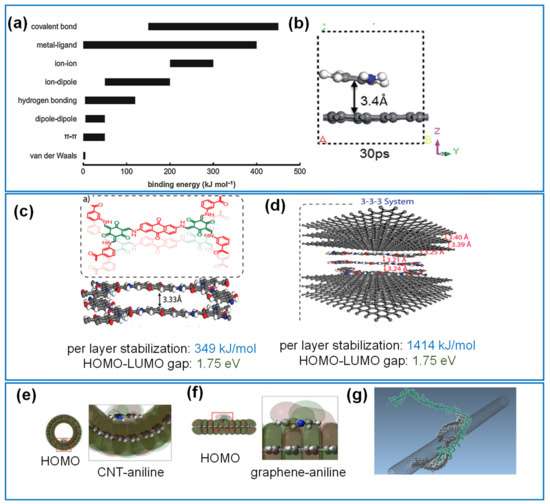
Covalent bonding of species onto the carbon substrate or chemisorption often leads to more chemically and thermally stable composites. The interaction energies vary between 100–400 kJ/mol, which is usually an order more than that for non-covalent interactions [54]. Covalent bonds, unlike weaker interactions, often form sp 3 structures from the pristine sp 2 configuration of the carbon substrate such as graphene [51], resulting in a less reversible structural change compared to noncovalent interactions. DFT-based quantum mechanical approaches commonly serve as adequate tools for approximating the formation and stability of covalent bonds in the composites, including bond dissociation energies, transition states during the grafting reactions on the surface of the carbon HOMO-LUMO gaps, electronic behavior and surface conformations for these adsorption scenarios.
Atomistic computational investigations of polymerization or chemical grafting on carbon substrates have been performed with small molecules such as pyrenes [62], diazonium cations [63], triazine [64] and biopolymers such as chitosan [65]. This form of bonding between components of a composite commonly requires a more involved fabrication process relative to non-covalently attached composites.
3.2. Fabrication methods
Numerous polymerization and deposition methods have been developed for capacitive composite electrodes with differences in the conditions that affect the successful deposition/polymerization and the process scalability. Further considerations include the microstructures and properties such as coverage, bonding, morphology and porosity of the composites.
The electropolymerization of redox active organic compounds has been the most widely used method. It applies an electrical energy to the electrode immersed in a monomer solution, which is simple, fast, versatile, environmentally benign, and applicable to most redox active polymeric materials. Changes in the polymerization conditions, e.g., monomer concentration, electrode potential, current density, and electrolyte pH will impact the morphology and properties of the polymer [66]. Each type of monomer has an optimal oxidation potential and current for homogeneous polymerization on the substrates. The techniques frequently used in polymerization include potential cycling, potentiostatic steps, and galvanostatic constant current [66]. The electropolymerization of redox active species starts with the oxidation of monomers at a specific potential, followed by the formation of cation radicals of monomers that react with adjacent monomers to form oligomeric products. The process continues during the elongation phase to finally form the polymer chain. The synthesis and doping in polymers are believed to take place simultaneously. The use of electric power supply and the small-scale production of material are the main limitations of electropolymerization.
In-situ chemical polymerization is also widely used to deposit functional redox active polymers on carbons. The polymerization occurs in solution containing monomers and an oxidizing agent. During the reaction, the monomers diffuse and adsorb on the substrate, and the polymerization is driven by the oxidizing agents [67]. Relatively strong chemical oxidants (initiator) are utilized, including ammonium peroxydisulfate (APS), permanganate or bichromate anions, ferric ions, Mg-H+, and hydrogen peroxide [68]. The reactions can occur in aqueous acidic and non-aqueous environments, depending on the solubility of the monomer. Different from electro polymerization, only chemical energy is consumed to produce the final products. However, safety issues can arise from the use of strong oxidizing agents in acid environment. The reaction conditions, e.g., the concentration ratio of monomer/oxidant and reaction environment such as the temperature and pH, etc., also need to be optimized to obtain a consistent and reproducible deposition [69].
Hydrothermal or solvothermal deposition methods have been applied to modify carbon substrate with small molecules or for polymerization. Different from the typical kinetically driven conditions, hydrothermal reactions are based on the thermodynamic control [70]. During both hydrothermal deposition and polymerization, organic molecules are added to the carbon substrate in an aqueous solution at elevated temperature and pressure [71]. The main advantage is the absence of additional chemical reactant such as oxidizing agent, catalysts or applied electrical energy to drive the reaction. The combined effect of high temperature and pressure provides a one-step process to produce composite electrode materials.
While polymeric materials are often deposited via electro- or chemical- polymerization, small redox active molecules can be deposited through direct deposition. One way is to modify the carbon substrate and/or the organic molecules with added functionalities such as polar groups [72] or sulfonate groups [73], so that the components can interact via hydrogen bonds, interactions leveraging the sp2 carbon structure of the substrate, or covalent bonds [73]. The other approach of applying organic compounds to carbon is through electrostatic self-assembly [29][74]. Other techniques that have been explored include vapor phase polymerization (VPP) and oxidative vapor phase polymerization (OVVP) [75]. These methods produce thin films of polymer on substrates and have advantages such as independence of the solubility of monomers, and the possibility of multiple monomers in one reaction chamber for synthesis of copolymers, which are promising for large scale manufacturing of functional and stable composite electrodes [76].
3.3 Capacitive organic-carbon composite electrodes
A significant fraction of composite electrodes in the literature use carbon substrates involving graphene [77] and CNTs, which reflects the current focus in research. Current state-of-the-art composite electrodes based on organic redox active materials draw from a growing list of materials. While the goals of developing composite electrodes are to increase the capacitances and energy densities, power and rate capability for capacitive electrodes are equally important. It is necessary to balance these parameters when designing and processing the capacitive electrodes to avoid developing just “mediocre batteries” [78].
Recently, Russell et.al. reported a highly pseudocapacitive organic network perylene diimide–hexaazatrinaphthylene (PHATN) system that possess high capacitance of 689 F/g, excellent stability over 50,000 cycles, and the highest rate capability of 75 A/g [79]. The keys for these successful performances are: (1) the selection of complementary electroactive components that expands the voltage range and thus the charge-storage capacity of the system; and (2) the contortion of the aromatic surface contributing to the pseudocapacitive behaviour, which opens space for electrolyte and ions movement for high rate. In addition, the customizability of COFs enables efficient and fast proton transfer at hydrophilic sites. Adsorbed water chains transport protons through the removal and formation of O-H bonds via the Grotthuss mechanism, promoting pseudocapacitive behavior [80].
Phenylenediamine (PD) molecules have shown promising capacitive performance on the surface of graphene. They can prevent the re-stacking of the nanosheets by increasing the interlayer spacing and contribute to the charge storage via reversible faradaic reactions. The fabrication techniques have significant influence on the overall electrochemical responses of the composite electrodes. For instance, reducing the oxygen groups of the GO substrate prior to the covalent bonding of p-PD gave different microstructures and lowered the capacitance from 316 F/g to 249 F/g.
Another example is CNT modified with carboxy (COOH) and amino (NH2) functionalized pyrene electropolymerized where COOH-Pyrene and NH2-Pyrene exhibited very different redox behavior on oxidized CNT. Although both had reversible redox peaks on their respective CVs, NH2-Pyrene-CNT had the most capacitive-like profile. Performance wise, the NH2-Pyrene-CNT had nearly twice the capacitance (210 F/g) of COOH-Pyrene-CNT (113 F/g) [24]. Since pyrene derivatives are abundant in industrial waste, applying these molecules in composite electrodes could lead to more sustainability toward lowering the carbon footprints.
4. Conclusions
The high-power densities, fast rate, and long charge-discharge life cycles of the redox active organic species for capacitive electrodes rely mostly on surface-confined processes. While Faradic redox reactions can significantly increase the capacitance and energy storage in organic-carbon composite electrodes, the surface and interfacial phenomena are important factors to consider for limiting diffusion limited processes. In addition, for future energy storage solutions, it is also important to take into account the low cost and high environmental sustainability of redox active organic species.
This entry is adapted from the peer-reviewed paper 10.3390/suschem2030024
References
- Afif, A.; Rahman, S.M.H.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852.
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: Boston, MA, USA, 1999; p. 685.
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163.
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854.
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782.
- Han, Y.; Ge, Y.; Chao, Y.; Wang, C.; Wallace, G.G. Recent progress in 2D materials for flexible supercapacitors. J. Energy Chem. 2018, 27, 57–72.
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54.
- Conway, B.E. Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548.
- Su, F.; Wu, Z.-S. A perspective on graphene for supercapacitors: Current status and future challenges. J. Energy Chem. 2021, 53, 354–357.
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794.
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19.
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloys Compd. 2018, 734, 89–111.
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356.
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199.
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816.
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12.
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
- Bakker, M.G.; Frazier, R.M.; Burkett, S.; Bara, J.E.; Chopra, N.; Spear, S.; Pan, S.; Xu, C. Perspectives on supercapacitors, pseudocapacitors and batteries. Nanomater. Energy 2012, 1, 136–158.
- Niu, Z.; Liu, L.; Zhou, W.; Chen, X.; Xie, S. Carbon Nanotube-Based Thin Films for Flexible Supercapacitors. In Nanocarbons for Advanced Energy Storage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 279–299.
- Balli, B.; Şavk, A.; Şen, F. Graphene and polymer composites for supercapacitor applications. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin Asiri, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 123–151.
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 387.
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34.
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano. 2010, 4, 1963–1970.
- Bachman, J.C.; Kavian, R.; Graham, D.J.; Kim, D.Y.; Noda, S.; Nocera, D.G.; Shao-Horn, Y.; Lee, S.W. Electrochemical polymerization of pyrene derivatives on functionalized carbon nanotubes for pseudocapacitive electrodes. Nat. Commun. 2015, 6, 7040.
- Ghosh, S.; An, X.; Shah, R.; Rawat, D.; Dave, B.; Kar, S.; Talapatra, S. Effect of 1-Pyrene Carboxylic-Acid Functionalization of Graphene on Its Capacitive Energy Storage. J. Phys. Chem. C 2012, 116, 20688–20693.
- Gao, P.; Chen, Z.; Zhao-Karger, Z.; Mueller, J.E.; Jung, C.; Klyatskaya, S.; Diemant, T.; Fuhr, O.; Jacob, T.; Behm, R.J.; et al. A Porphyrin Complex as a Self-Conditioned Electrode Material for High-Performance Energy Storage. Angew. Chem. Int. Ed. Engl. 2017, 56, 10341–10346.
- Agboola, B.O.; Ozoemena, K.I. Synergistic enhancement of supercapacitance upon integration of nickel (II) octa [(3,5-biscarboxylate)-phenoxy] phthalocyanine with SWCNT-phenylamine. J. Power Sources 2010, 195, 3841–3848.
- Chidembo, A.T.; Ozoemena, K.I. Electrochemical Capacitive Behaviour of Multiwalled Carbon Nanotubes Modified with Electropolymeric Films of Nickel Tetraaminophthalocyanine. Electroanalysis 2010, 22, 2529–2535.
- Chidembo, A.T.; Ozoemena, K.I.; Agboola, B.O.; Gupta, V.; Wildgoose, G.G.; Compton, R.G. Nickel(ii) tetra-aminophthalocyanine modified MWCNTs as potential nanocomposite materials for the development of supercapacitors. Energy Environ. Sci. 2010, 3, 228–236.
- Navarro-Suárez, A.M.; Carretero-González, J.; Rojo, T.; Armand, M. Poly(quinone-amine)/nanocarbon composite electrodes with enhanced proton storage capacity. J. Mater. Chem. A 2017, 5, 23292–23298.
- Tabor, D.P.; Gómez-Bombarelli, R.; Tong, L.; Gordon, R.G.; Aziz, M.J.; Aspuru-Guzik, A. Mapping the frontiers of quinone stability in aqueous media: Implications for organic aqueous redox flow batteries. J. Mater. Chem. A 2019, 7, 12833–12841.
- Song, B.; Choi, J.I.; Zhu, Y.; Geng, Z.; Zhang, L.; Lin, Z.; Tuan, C.-C.; Moon, K.-S.; Wong, C.-P. Molecular Level Study of Graphene Networks Functionalized with Phenylenediamine Monomers for Supercapacitor Electrodes. Chem. Mater. 2016, 28, 9110–9121.
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251.
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473.
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904.
- Lv, W.; Li, Z.; Deng, Y.; Yang, Q.-H.; Kang, F. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges. Energy Storage 2016, 2, 107–138.
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665.
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531.
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966.
- Wang, J.-G.; Liu, H.; Zhang, X.; Shao, M.; Wei, B. Elaborate construction of N/S-co-doped carbon nanobowls for ultrahigh-power supercapacitors. J. Mater. Chem. A 2018, 6, 17653–17661.
- Yang, L.; Wu, D.; Wang, T.; Jia, D. B/N-Codoped Carbon Nanosheets Derived from the Self-Assembly of Chitosan–Amino Acid Gels for Greatly Improved Supercapacitor Performances. ACS Appl. Mater. Interfaces 2020, 12, 18692–18704.
- Tanguy, N.R.; N’Diaye, J.; Arjmand, M.; Lian, K.; Yan, N. Facile one-pot synthesis of water-dispersible phosphate functionalized reduced graphene oxide toward high-performance energy storage devices. Chem. Commun. 2020, 56, 1373–1376.
- Genovese, M.; Wu, H.; Virya, A.; Li, J.; Shen, P.; Lian, K. Ultrathin all-solid-state supercapacitor devices based on chitosan activated carbon electrodes and polymer electrolytes. Electrochim. Acta 2018, 273, 392–401.
- Kharisov, B.I.; Kharissova, O.V. General Data on Carbon Allotropes. In Carbon Allotropes: Metal-Complex Chemistry, Properties and Applications; Kharisov, B.I., Kharissova, O.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–8.
- N’Diaye, J.; Hyun Chang, J.; Lian, K. The Capacitive Behavior of Polyluminol on Carbon Nanotubes Electrodes. ChemElectroChem 2019, 6, 5454–5461.
- Wu, H.; Genovese, M.; Ton, K.; Lian, K. A Comparative Study of Activated Carbons from Liquid to Solid Polymer Electrolytes for Electrochemical Capacitors. J. Electrochem. Soc. 2019, 166, A821–A828.
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947.
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27.
- Weinstein, L.; Dash, R. Supercapacitor carbons. Mater. Today 2013, 16, 356–357.
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855.
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519.
- Raimondo, M.; Naddeo, C.; Vertuccio, L.; Bonnaud, L.; Dubois, P.; Binder, W.H.; Sorrentino, A.; Guadagno, L. Multifunctionality of structural nanohybrids: The crucial role of carbon nanotube covalent and non-covalent functionalization in enabling high thermal, mechanical and self-healing performance. Nanotechnology 2020, 31, 225708.
- Cho, Y.; Cho, W.J.; Youn, I.S.; Lee, G.; Singh, N.J.; Kim, K.S. Density functional theory based study of molecular interactions, recognition, engineering, and quantum transport in π molecular systems. Acc. Chem. Res. 2014, 47, 3321–3330.
- Jonathan, W. Steed, J.L.A. Supramolecular Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013.
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. NPJ Comput. Mater. 2016, 2, 15011.
- Elbaz, Y.; Furman, D.; Caspary Toroker, M. Modeling Diffusion in Functional Materials: From Density Functional Theory to Artificial Intelligence. Adv. Funct. Mater. 2020, 30, 1–18.
- Benda, R.; Zucchi, G.; Cancès, E.; Lebental, B. Insights into the π–π interaction driven non-covalent functionalization of carbon nanotubes of various diameters by conjugated fluorene and carbazole copolymers. J. Chem. Phys. 2020, 152, 064708.
- Mohammed, A.K.; Vijayakumar, V.; Halder, A.; Ghosh, M.; Addicoat, M.; Bansode, U.; Kurungot, S.; Banerjee, R. Weak Intermolecular Interactions in Covalent Organic Framework-Carbon Nanofiber Based Crystalline yet Flexible Devices. ACS Appl. Mater. Interfaces 2019, 11, 30828–30837.
- Duan, Y.; Liu, J.; Zhang, Y.; Wang, T. First-principles calculations of graphene-based polyaniline nano-hybrids for insight of electromagnetic properties and electronic structures. RSC Adv. 2016, 6, 73915–73923.
- Ahn, D.H.; Park, C.; Song, J.W. Predicting whether aromatic molecules would prefer to enter a carbon nanotube: A density functional theory study. J. Comput. Chem. 2020, 41, 1261–1270.
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25.
- Boukhvalov, D.W. DFT modeling of the covalent functionalization of graphene: From ideal to realistic models. RSC Adv. 2013, 3, 7150–7159.
- Berisha, A. Interactions between the aryldiazonium cations and graphene oxide: A DFT study. J. Chem. 2019, 2019, 1–5.
- Oliveira, O.N.; Aoki, P.H.B.; Pavinatto, F.J.; Constantino, C.J.L. Controlled Architectures in LbL Films for Sensing and Biosensing. In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 951–983.
- Mokhtari, A.; Harismah, K.; Mirzaei, M. Covalent addition of chitosan to graphene sheets: Density functional theory explorations of quadrupole coupling constants. Superlattices Microstruct. 2015, 88, 56–61.
- Gabe, A.; Mostazo-López, M.J.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Synthesis of conducting polymer/carbon material composites and their application in electrical energy storage. In Hybrid Polymer Composite Materials; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 173–209.
- Si, K.; Guo, X.Q.; Qiu, K.Y. Initiation Mechanism of Radical Polymerization Using Ammonium Persulfate and Polymerizable Amine Redox Initiators. J. Macromol. Sci. Part A 1995, 32, 1149–1159.
- Mart, H. Oxidative polycondensation reaction. Des. Monomers Polym. 2012, 9, 551–588.
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52.
- Li, S.; Chen, Y.; He, X.; Mao, X.; Zhou, Y.; Xu, J.; Yang, Y. Modifying Reduced Graphene Oxide by Conducting Polymer Through a Hydrothermal Polymerization Method and its Application as Energy Storage Electrodes. Nanoscale Res. Lett 2019, 14, 226.
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236.
- Lu, X.; Li, L.; Song, B.; Moon, K.-S.; Hu, N.; Liao, G.; Shi, T.; Wong, C. Mechanistic investigation of the graphene functionalization using p-phenylenediamine and its application for supercapacitors. Nano Energy 2015, 17, 160–170.
- N’Diaye, J.; Elshazly, M.; Lian, K. Capacitive Charge Storage of Tetraphenylporphyrin Sulfonate- CNT Composite Electrodes. Electrochim. Acta 2021, 389, 138593.
- Park, Y.T.; Grunlan, J.C. Carbon Nanotube-Based Multilayers. In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 595–612.
- Bhattacharyya, D.; Howden, R.M.; Borrelli, D.C.; Gleason, K.K. Vapor phase oxidative synthesis of conjugated polymers and applications. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1329–1351.
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.S.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing polymer surfaces via vapor deposition. Mater. Today 2010, 13, 26–33.
- Li, Q.; Horn, M.; Wang, Y.; MacLeod, J.; Motta, N.; Liu, J. A Review of Supercapacitors Based on Graphene and Redox-Active Organic Materials. Materials 2019, 12, 703.
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917.
- Russell, J.C.; Posey, V.A.; Gray, J.; May, R.; Reed, D.A.; Zhang, H.; Marbella, L.E.; Steigerwald, M.L.; Yang, Y.; Roy, X.; et al. High-performance organic pseudocapacitors via molecular contortion. Nat. Mater. 2021, 20, 1136–1141.
- Wu, X.; Hong, J.J.; Shin, W.; Ma, L.; Liu, T.; Bi, X.; Yuan, Y.; Qi, Y.; Surta, T.W.; Huang, W.; et al. Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries. Nat. Energy 2019, 4, 123–130.
