Photodeformable azobenzene (azo) polymers are a class of smart polymers that can efficiently convert light energy into mechanical power, holding great promise in various photoactuating applications. They are typically of crosslinked polymer networks with highly oriented azo mesogens embedded inside. Upon exposure to the light of appropriate wavelength, they experience dramatic order parameter change following the configuration change of the azo units. This could result in the generation and accumulation of the gradient microscopic photomechanical force in the crosslinked polymer networks, thus leading to their macroscopic deformation. Most of the presently developed photodeformable azo polymers have stable chemically crosslinked networks, which make their reprocessing impossible, thus limiting their large scale applications. To solve this problem, reprocessable photodeformable azo polymers have been recently developed by introducing dynamic crosslinking networks (including physically crosslinked and dynamic covalent bond-crosslinked ones) into their structures. In addition, some uncrosslinked photodeformable azo polymers have also been reported and constitute one special type of reprocessable photodeformable azo polymers, whose photodeformation behaviors are mainly induced by the selective reorientation of the azo moieties (become perpendicular to the polarization direction of the polarized light) under the irradiation of either polarized blue light or interfering polarized light.
- azobenzene polymers
- photodeformable
- reprocessable
- dynamic crosslinking networks
1. Introduction
Photodeformable azobenzene (azo) polymers are a class of smart functional polymers that can convert light energy into macroscopic displacement or deformation (i.e., photomechanical effects) [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15]. As a photoswitchable chromophore, azo unit can reversibly change the conformation between two structural configurations (i.e., trans and cis isomers) with high quantum efficiency and fatigue resistance [16][17]. In particular, the photoisomerization of an azo unit from the rod-like trans-form to the bent cis-form can lead to its large size (or length) alteration from 0.9 to 0.55 nm, which can lead to dramatic changes in the alignment order of azo mesogens and the conformation of their surrounding polymer chains, thus resulting in the generation of microscopic force inside azo polymers and their photomechanical effects. They can show various macroscopic photodeformation behaviors under the irradiation of appropriate light such as contraction, bending, twisting, oscillation, rotation and translational motion. They have shown great potential in a wide range of actuating applications such as soft robots, artificial muscles, and microfluidic devices.
It has also been well demonstrated that the presence of an appropriate crosslinking degree in the photodeformable azo polymer actuators is essential for them to transport the mechanical force generated by the photoisomerization of azo mesogens to the whole system and induce the reversible macroscopic motion [4][18][19][20][21]. Among all the presently developed photodeformable azo polymers, most of them have stable chemical crosslinking structures. Although they could exhibit good photomechanical and mechanical properties, they have some obvious drawbacks such as the typical complicated crosslinking processes, low flexibility in fabrication, and less freedom for further processing and recycling. To address these issues, a number of reprocessable photodeformable azo polymers have been developed on the basis of different design strategies [18][20][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75]. The outline of this entry is shown in Figure 1.
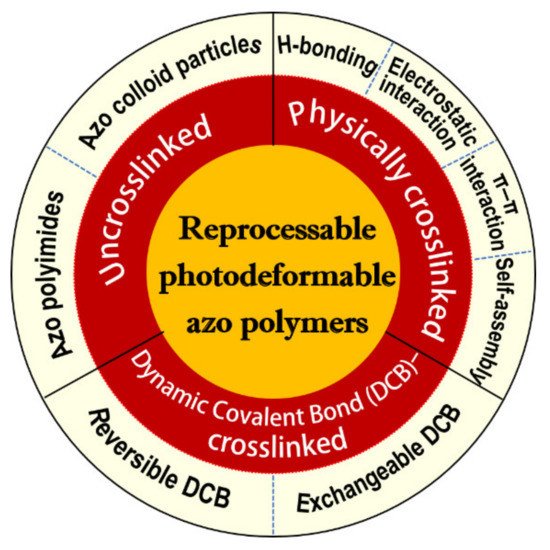
2. Reprocessable Physically Crosslinked Photodeformable Azo Polymers
The reprocessable physically crosslinked photodeformable azo polymers are photodeformable polymers with crosslinked networks that are formed through various supramolecular non-covalent interactions including hydrogen bonding (H-bonding) [18][20][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36], electrostatic interaction [37][38], π-π interaction [39][40], and other self-assembly-induced interactions [41][42][43][44][45][46][47][48][49].
2.1. Hydrogen Bonding (H-bonding) Interactions
Hydrogen bonds (H-bonds) are readily formed between a donor with an available acidic hydrogen atom and an acceptor carrying a nonbonding lone pair of electrons. They are highly selective and directional and can show environmental stimulus-responsivity owing to the dependence of their strength on the solvent and temperature. Therefore, recent years have witnessed tremendous interest in the application of H-bonds for the construction of various supramolecular polymer systems including supramolecular azo polymers [76][77][78][79].
2.1.1. H-Bond-Crosslinked Photodeformable Side-Chain Azo Polymers
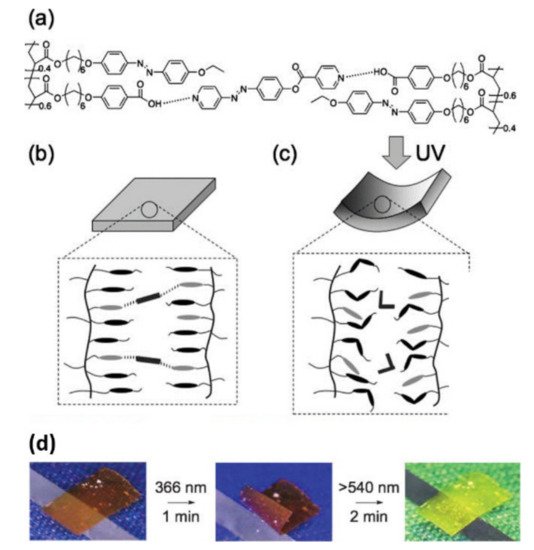
In 2008, Ikeda and coworkers reported the first example of a supramolecular photomechanical system based on the H-bonded physically crosslinked azo LC polymer (LCP) film [22]. A side-chain polyacrylate with pendant benzoic acid units and ethoxyl group-terminated azo groups (PAAC) and an azo crosslinker with a pyridine unit at its both ends (PEAP) were used for such a purpose (Figure 2a). A free-standing physically crosslinked film with preferentially aligned azo mesogens along the film surface was fabricated by putting a melt mixture of PAAC and PEAP between two NaCl plates with rubbing treatment (Figure 2b). Under the irradiation of UV/visible light, the azo polymer film showed photoinduced bending and unbending (Figure 2c,d).
2.1.2. H-Bond-Crosslinked Photodeformable Main-Chain Azo Polymers
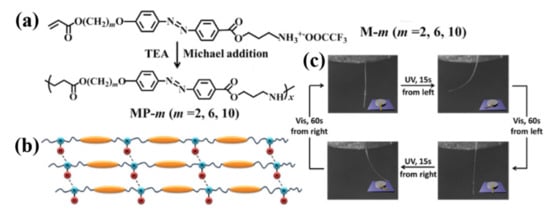
In 2013, our group developed a versatile approach for preparing physically crosslinked photodeformable main-chain azo LCPs with both ester and secondary amino units in their backbones (Figure 3a,b) [18]. It involves first the synthesis of a series of acrylate-type azo monomers bearing an amino terminal group and their subsequent Michael addition polymerization under the mild condition (Figure 3a). These main-chain azo polymers showed high thermal stability, relatively low Tg (down to 30 °C as determined by DSC), a broad temperature range of smectic C mesophases, and reversible photoresponsivity. Their uniaxially oriented fibers with a high alignment order of azo mesogens along the fiber axes and easily tunable diameters were readily fabricated by using the simple melt spinning method. They showed rapid and reversible photoinduced deformation even at close to ambient temperature (Figure 3c). They could produce photoinduced stress around 240 kPa under the UV light irradiation (150 mW/cm2), which is close to the stress generated by the chemically crosslinked azo LCP films [80] and fibers [81] as well as that of the human muscles (around 300 kPa) [80][81].
2.2. Electrostatic Interaction
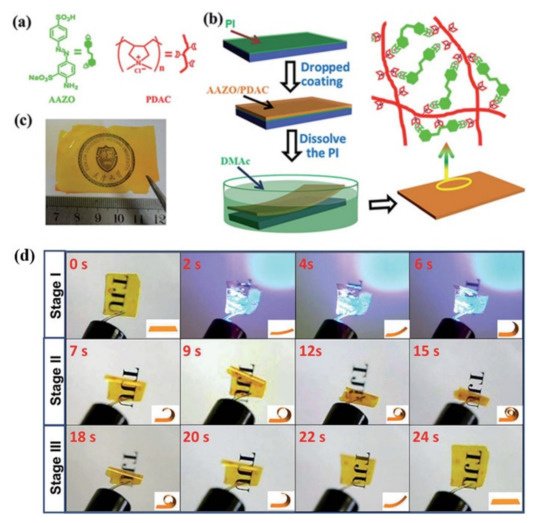
The electrostatic interaction involves ions, dipoles, and induced dipoles. Recent years have witnessed considerable interest in their application in the design of various advanced functional supramolecular polymers [78][79]. It has also been successfully utilized to construct physically crosslinked azo polymer photoactuators by Feng and coworkers [37][38]. The first such supramolecular crosslinked azo polymer photoactuator was prepared by the electrostatic interaction between a photochromic azo compound with disulfonic groups (AAzo) and a cationic polyelectrolyte (PDAC) (Figure 4a–c) [37]. Under the optimal weight ratio of AAzo and PDAC (1:4), the unoriented supramolecularly self-assembled azo polymer film exhibited a large deformation under light illumination (Stage I) and continue to deform when the light was off (Stage II), along with a spontaneous shape recovery (Stage III) (Figure 4d). This photomechanical deformation demonstrated that the crosslinking by electrostatic interaction is able to convert the microscopic motion of the azo moieties into a macroscopic change of the film.
2.3. π–π Interaction
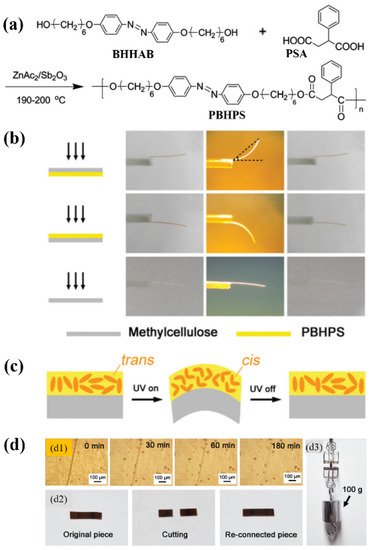
π–π stacking interaction is a kind of weak interaction often existing between the aromatic rings, which has already been applied for the construction of supramolecular polymer systems [79]. To date, it has also been successfully used for developing physically crosslinked main-chain azo polymer photoactuators [39][40]. Wang and coworkers prepared a thermo- and photo-responsive main-chain azo LC polyester (namely PBHPS) via the polyesterification of 4,4′-bis(6-hydroxyhexyloxy)azobenzene (BHHAB) and 2-phenylsuccinic acid (PSA) (Figure 5a) [39]. PBHPS showed a relatively low Tg (26.9 °C by DSC) and a smectic LC phase. Strong π-π interaction was found to exist between its adjacent phenyl rings or between its side group and mesogenic unit. The π–π interaction-induced physical crosslinking imparted the unoriented PBHPS/methylcellulose (MC) bilayer film with reversible photoinduced bending behaviors (Figure 5b), which was attributed to the UV light-induced volume expansion of the PBHPS layer (Figure 5c). Moreover, it also endowed the PBHPS film with good shape memory and self-healing properties (Figure 5d). Later on, the same group also developed a series of novel LC copolyesters (namely P(BH-co-BPn)PS) with amphi-mesogenic units (including azo and biphenyl groups) via the one-pot melt polycondensation of BHHAB, 4,4′-bis(6-hydroxyhexyloxy)biphenyl (BHHBP), and PSA [40]. The resulting copolyesters showed good thermal stability, low Tg (around 25 °C by DSC), and smectic LC phases.
2.4. Self-Assembly-Induced Physical Crosslinking
So far, some reprocessable azo polymer photoactuators have also been developed via the self-assembly-induced physical crosslinking, which mainly include those physically crosslinked ones based on the self-assembly of mesogens [41][42][43][44][45][46][47][48] and those based on the microphase separation of the azo block copolymers [49].
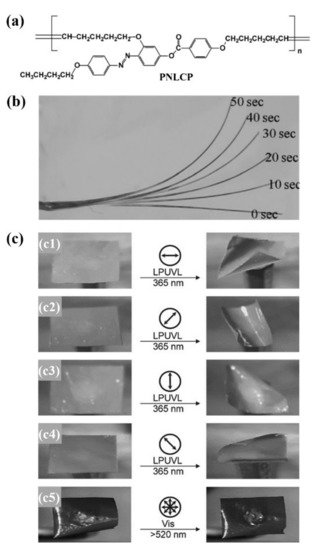
Lee and Jeong’s group reported the fabrication of some photochromic 3D actuators from the uncrosslinked azo LCEs with self-assembled LC phase-induced physical crosslinking structures [41][42][43]. They prepared a main-chain polymer with azo mesogen at the flank via the acyclic diene metathesis polymerization (ADMET) of a novel photochromic LC monomer (PNLCM) (Figure 6a) [41][42]. The azo polymer had a Mw,GPC of 1.21 × 104 g/mol, a Tg of 24 °C, and a nematic LC phase (Tcl = 145 °C). The uniaxially oriented azo polymer fiber fabricated via the melt spinning method bent toward the incident UV light (Figure 6b). The free-standing azo polymer film (of 20 μm thickness) prepared via the solution casting method also bent toward the light source upon exposure to both nonpolarized and polarized UV light. In the case of the nonpolarized UV light, the four corners of the film bent upward toward the light source at room temperature within 2 min owing to both the conformational transformation of polymer chains from the extended to the random-coil conformation (caused by trans to cis azo photoisomerization) and different degrees of the molecular orientational order change between the upper and lower layers of the film. In the case of the polarized UV light, only two sides of the film bent upward, and the bending direction had an approximately 45° angle to the direction of the polarized light (Figure 6c).
3. Reprocessable Photodeformable Azo Polymers with Dynamic Covalent Bond (DCB)-Crosslinked Networks
Despite the significant progress made in the physically crosslinked photodeformable azo polymers, they typically have some disadvantages including their typical relatively lower thermal and anti-solvent capability. To address these issues, two types of reprocessable chemically crosslinked photodeformable azo polymers have been mainly developed by applying DCBs [50][51][52][53][54][55][56][57][58][59][60], which contain reversible chemical crosslinking [50][51][52] and rearrangeable (or exchangeable) chemical crosslinking [53][54][55][56][57][58][59][60], respectively. They have proven to show the advantages of both the chemically and physically crosslinked systems (i.e., high stability and good reprocessability).
DCBs are covalent bonds that can switch or exchange between two or several molecules under the appropriate external stimuli [82][83][84][85]. They have garnered tremendous interest in the field of polymer science because the incorporation of DCBs into polymers can endow them with many fascinating properties such as reprocessability, self-healability, shape-memory, high toughness, and various stimulus-responsivity. Following the milestone work by Leibler and coworkers in the preparation of crosslinked polymers with rearrangeable networks (or briefly “vitrimers”) through the transesterification between epoxy and ester bonds [86][87], many highly reprocessable crosslinked polymers have been developed by using DCB-forming reactions, including the reversible ones (e.g., redox-based thiol-disulfide switch and thermally reversible Diels–Alder (DA) reaction) and exchangeable ones (e.g., catalytically/thermally controlled transesterification, acid or base-catalyzed transthioesterfication, transimination reaction with or without catalysts, and thermo/photoinduced disulfide exchange) [82][83][84][85].
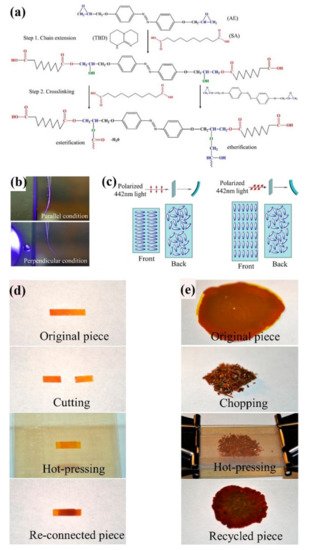
4. Some Other Reprocessable Uncrosslinked Photodeformable Azo Polymers
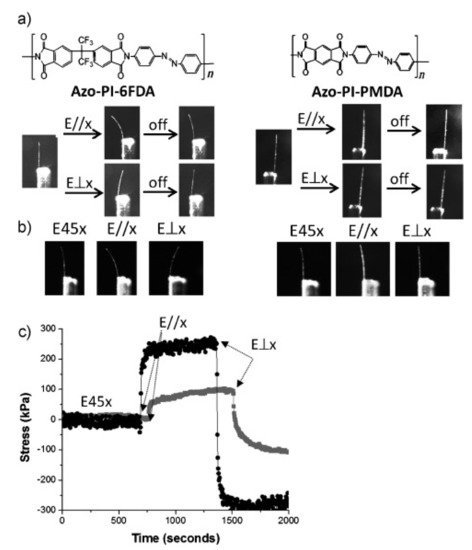
5. Conclusions and Outlook
This entry is adapted from the peer-reviewed paper 10.3390/molecules26154455
References
- Li, M.-H.; Keller, P. Artificial muscles based on liquid crystal elastomers. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2006, 364, 2763–2777.
- Ikeda, T.; Mamiya, J.-I.; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528.
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387.
- Ikeda, T.; Ube, T. Photomobile polymer materials: From nano to macro. Mater. Today 2011, 14, 480–487.
- Yu, H.; Ikeda, T. Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011, 23, 2149–2180.
- Yang, H.; Ye, G.; Wang, X.; Keller, P. Micron-sized liquid crystalline elastomer actuators. Soft Matter 2011, 7, 815–823.
- Jiang, H.; Li, C.; Huang, X. Actuators based on liquid crystalline elastomer materials. Nanoscale 2013, 5, 5225–5240.
- Ube, T.; Ikeda, T. Photomobile polymer materials with crosslinked liquid-crystalline structures: Molecular design, fabrication, and functions. Angew. Chem. Int. Ed. 2014, 53, 10290–10299.
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098.
- Bushuyev, O.S.; Aizawa, M.; Shishido, A.; Barrett, C.J. Shape-shifting azo dye polymers: Towards sun-light-driven molecular devices. Macromol. Rapid Commun. 2018, 39, 1700253.
- Jiang, Z.-C.; Xiao, Y.; Zhao, Y. Shining light on liquid crystal polymer networks: Preparing, reconfiguring, and driving soft actuators. Adv. Opt. Mater. 2019, 7, 1900262.
- Ube, T.; Ikeda, T. Photomobile polymer materials with complex 3D deformation, continuous motions, self-regulation, and enhanced processability. Adv. Opt. Mater. 2019, 7, 1900380.
- Pang, X.; Lv, J.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv. Mater. 2019, 31, 1904224.
- McCracken, J.M.; Donovan, B.R.; White, T.J. Materials as machines. Adv. Mater. 2020, 32, 1906564.
- Chen, M.; Liang, S.; Liu, C.; Liu, Y.; Wu, S. Reconfigurable and recyclable photoactuators based on azobenzene-containing polymers. Front. Chem. 2020, 8, 706.
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925.
- Chang, V.Y.; Fedele, C.; Priimagi, A.; Shishido, A.; Barrett, C.J. Photoreversible soft azo dye materials: Toward optical control of bio-interfaces. Adv. Opt. Mater. 2019, 7, 1900091.
- Fang, L.; Zhang, H.T.; Li, Z.; Zhang, Y.; Zhang, Y.Y.; Zhang, H. Synthesis of reactive azobenzene main-chain liquid crystalline polymers via Michael addition polymerization and photomechanical effects of their supramolecular hydrogen-bonded fibers. Macromolecules 2013, 46, 7650–7660.
- Fang, L.; Han, G.; Zhang, J.; Zhang, H.T.; Zhang, H. Synthesis of well-defined easily crosslinkable azobenzene side-chain liquid crystalline polymers via reversible addition-fragmentation chain transfer polymerization and photomechanical properties of their post-crosslinked fibers. Eur. Polym. J. 2015, 69, 592–604.
- Nie, J.; Liu, X.; Yan, Y.; Zhang, H. Supramolecular hydrogen-bonded photodriven actuators based on an azobenzene-containing main-chain liquid crystalline poly(ester-amide). J. Mater. Chem. C 2017, 5, 10391–10398.
- Dong, H.; Liu, G.; Zhang, H. Preparation of photodeformable azobenzene polymer fibers by postcrosslinking strategy: Understanding the structure-property relationship. Eur. Polym. J. 2020, 135, 109863.
- Mamiya, J.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65.
- Ozawa, T.; Kondo, M.; Mamiya, J.; Ikeda, T. Enhancement of mechanical stability in hydrogenbonded photomobile materials with chemically modified single-walled carbon nanotubes. J. Mater. Chem. C 2014, 2, 2313–2315.
- Wang, J.; Huang, S.; Zhang, Y.; Liu, J.; Yu, M.; Yu, H. Hydrogen bond enhances photomechanical swing of liquid-crystalline polymer bilayer films. ACS Appl. Mater. Interfaces 2021, 13, 6585–6596.
- Yan, M.; Tang, J.; Xie, H.-L.; Ni, B.; Zhang, H.-L.; Chen, E.-Q. Self-healing and phase behavior of liquid crystalline elastomer based on a block copolymer constituted of a side-chain liquid crystalline polymer and a hydrogen bonding block. J. Mater. Chem. C 2015, 3, 8526–8534.
- Ni, B.; Xie, H.-L.; Tang, J.; Zhang, H.-L.; Chen, E.-Q. A self-healing photoinduced-deformable material fabricated by liquid crystalline elastomers using multivalent hydrogen bonds as cross-linkers. Chem. Commun. 2016, 52, 10257–10260.
- Si, Q.; Feng, Y.; Yang, W.; Fu, L.; Yan, Q.; Dong, L.; Long, P.; Feng, W. Controllable and stable deformation of a self-healing photo-responsive supramolecular assembly for an optically actuated manipulator arm. ACS Appl. Mater. Interfaces 2018, 10, 29909–29917.
- Yu, C.-Y.; Mu, J.-H.; Fu, Y.-L.; Zhang, Y.-C.; Han, J.-S.; Zhao, R.-Y.; Zhao, J.; Wang, Z.-H.; Zhao, Z.-C.; Li, W.-J.; et al. Azobenzene based Photo-responsive mechanical actuator fabricated by intermolecular H-bond interaction. Chin. J. Polym. Sci. 2021, 39, 417–424.
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H. NIR-vis-UV light-responsive actuator films of polymer-dispersed liquid crystal/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501.
- Zhou, L.; Liu, Q.; Lv, X.; Gao, L.; Fang, S.; Yu, H. Photoinduced triple shape memory polyurethane enabled by doping with azobenzene and GO. J. Mater. Chem. C 2016, 4, 9993–9997.
- Xiong, Y.; Zhang, L.; Weis, P.; Naumov, P.; Wu, S. A solar actuator based on hydrogen-bonded azopolymers for electricity generation. J. Mater. Chem. A 2018, 6, 3361–3366.
- Wang, Z.-Z.; Zhang, H.-Q. Synthesis of an azobenzene-containing main-chain crystalline polymer and photodeformation behaviors of its supramolecular hydrogen-bonded fibers. Chin. J. Polym. Sci. 2020, 38, 37–44.
- Wie, J.J.; Wang, D.H.; Lee, K.M.; White, T.J.; Tan, L.-S. The contribution of hydrogen bonding to the photomechanical response of azobenzene-functionalized polyamides. J. Mater. Chem. C 2018, 6, 5964–5974.
- Ban, J.; Mu, L.; Yang, J.; Chen, S.; Zhuo, H. New stimulus-responsive shape-memory polyurethanes capable of UV light-triggered deformation, hydrogen bond-mediated fixation, and thermal-induced recovery. J. Mater. Chem. A 2017, 5, 14514–14518.
- Li, S.; Han, G.; Zhang, W. Concise synthesis of photoresponsive polyureas containing bridged azobenzenes as visible-light-driven actuators and reversible photopatterning. Macromolecules 2018, 51, 4290–4297.
- Li, S.; Tu, Y.; Bai, H.; Hibi, Y.; Wiesner, L.W.; Pan, W.; Wang, K.; Giannelis, E.P.; Shepherd, R.F. Simple synthesis of elastomeric photomechanical switches that self-heal. Macromol. Rapid Commun. 2019, 40, 1800815.
- Qin, C.; Feng, Y.; Luo, W.; Cao, C.; Hu, W.; Feng, W. A supramolecular assembly of cross-linked azobenzene/polymers for a high-performance light-driven actuator. J. Mater. Chem. A 2015, 3, 16453–16460.
- Qin, C.; Feng, Y.; An, H.; Han, J.; Cao, C.; Feng, W. Tetracarboxylated azobenzene/polymer supramolecular assemblies as high-performance multiresponsive actuators. ACS Appl. Mater. Interfaces 2017, 9, 4066–4073.
- Zhong, H.-Y.; Chen, L.; Yang, R.; Meng, Z.-Y.; Ding, X.-M.; Liu, X.-F.; Wang, Y.-Z. Azobenzene-containing liquid crystalline polyester with π-π interactions: Diverse thermo- and photo-responsive behaviours. J. Mater. Chem. C 2017, 5, 3306–3314.
- Zhong, H.-Y.; Chen, L.; Liu, X.-F.; Yang, R.; Wang, Y.-Z. Novel liquid crystalline copolyester containing amphi-mesogenic units toward multiple stimuliresponse behaviors. J. Mater. Chem. C 2017, 5, 9702–9711.
- Choi, H.J.; Jeong, K.-U.; Chien, L.-C.; Lee, M.-H. Photochromic 3-dimensional actuator based on an uncrosslinked liquid crystal elastomer. J. Mater. Chem. 2009, 19, 7124–7129.
- Kim, D.-Y.; Lee, S.-A.; Choi, H.J.; Chien, L.-C.; Lee, M.-H.; Jeong, K.-U. Reversible actuating and writing behaviours of a head-to-side connected main-chain photochromic liquid crystalline polymer. J. Mater. Chem. C 2013, 1, 1375–1382.
- Kim, D.-Y.; Shin, S.; Yoon, W.-J.; Choi, Y.-J.; Hwang, J.-K.; Kim, J.-S.; Lee, C.-R.; Choi, T.-L.; Jeong, K.-U. From smart denpols to remote-controllable actuators: Hierarchical superstructures of azobenzene-based polynorbornenes. Adv. Funct. Mater. 2017, 27, 1606294.
- Hosono, N.; Kajitani, T.; Fukushima, T.; Ito, K.; Sasaki, S.; Takata, M.; Aida, T. Large-area three-dimensional molecular ordering of a polymer brush by one-step processing. Science 2010, 330, 808–811.
- Lv, J.A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184.
- Xu, B.; Zhu, C.; Qin, L.; Wei, J.; Yu, Y. Light-directed liquid manipulation in flexible bilayer microtubes. Small 2019, 15, 1901847.
- Pang, X.; Qin, L.; Xu, B.; Liu, Q.; Yu, Y. Ultralarge contraction directed by light-driven unlocking of prestored strain energy in linear liquid crystal polymer fibers. Adv. Funct. Mater. 2020, 30, 2002451.
- Chen, M.; Yao, B.; Kappl, M.; Liu, S.; Yuan, J.; Berger, R.; Zhang, F.; Butt, H.-J.; Liu, Y.; Wu, S. Entangled azobenzene-containing polymers with photoinduced reversible solid-to-liquid transitions for healable and reprocessable photoactuators. Adv. Funct. Mater. 2020, 30, 1906752.
- Petr, M.; Katzman, B.-A.; DiNatale, W.; Hammond, P.T. Synthesis of a new, low-Tg siloxane thermoplastic elastomer with a functionalizable backbone and its use as a rapid, room temperature photoactuator. Macromolecules 2013, 46, 2823–2832.
- Han, G.; Nie, J.; Zhang, H. Facile preparation of recyclable photodeformable azobenzene polymer fibers with chemically crosslinked networks. Polym. Chem. 2016, 7, 5088–5092.
- Guo, C.; Gao, J.; Ma, S.; Zhang, H. Efficient preparation of chemically crosslinked recyclable photodeformable azobenzene polymer fibers with high processability and reconstruction ability via a facile post-crosslinking method. Eur. Polym. J. 2020, 139, 109998.
- Jiang, Z.-C.; Xiao, Y.-Y.; Yin, L.; Han, L.; Zhao, Y. “Self-lockable” liquid crystalline Diels–Alder dynamic network actuators with room temperature programmability and solution reprocessability. Angew. Chem. Int. Ed. 2020, 59, 4925–4931.
- Kawasaki, K.; Ube, T.; Ikeda, T. Remoldable crosslinked liquid-crystalline polysiloxane with side chain mesogens based on exchangeable crosslinks. Mol. Cryst. Liq. Cryst. 2015, 614, 62–66.
- Ube, T.; Kawasaki, K.; Ikeda, T. Photomobile liquid-crystalline elastomers with rearrangeable networks. Adv. Mater. 2016, 28, 8212–8217.
- Tsunoda, H.; Kawasaki, K.; Ube, T.; Ikeda, T. Liquid-crystalline elastomer photoactuator with photorearrangeable network structures. Mol. Cryst. Liq. Cryst. 2018, 662, 61–67.
- Matsushita, M.; Kawasaki, K.; Ube, T.; Ikeda, T. Remolding of photoresponsive polymer materials by means of dynamic covalent bonds in a main chain. Mol. Cryst. Liq. Cryst. 2018, 676, 17–23.
- Li, Y.; Rios, O.; Keum, J.K.; Chen, J.; Kessler, M.R. Photoresponsive liquid crystalline epoxy networks with shape memory behavior and dynamic ester bonds. ACS Appl. Mater. Interfaces 2016, 8, 15750–15757.
- Li, Y.; Zhang, Y.; Rios, O.; Keum, J.K.; Kessler, M.R. Photo-responsive liquid crystalline epoxy networks with exchangeable disulfide bonds. RSC Adv. 2017, 7, 37248–37254.
- Lu, X.; Guo, S.; Tong, X.; Xia, H.; Zhao, Y. Tunable photocontrolled motions using stored strain energy in malleable azobenzene liquid crystalline polymer actuators. Adv. Mater. 2017, 29, 1606467.
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-crystalline dynamic networks doped with gold nanorods showing enhanced photocontrol of actuation. Adv. Mater. 2018, 30, 1706597.
- Lee, K.M.; Wang, D.H.; Koerner, H.; Vaia, R.A.; Tan, L.-S.; White, T.J. Enhancement of photogenerated mechanical force in azobenzene-functionalized polyimides. Angew. Chem. Int. Ed. 2012, 51, 4117–4121.
- White, T.J. Light to work transduction and shape memory in glassy, photoresponsive macromolecular systems: Trends and opportunities. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 877–880.
- Wang, D.H.; Wie, J.J.; Lee, K.M.; White, T.J.; Tan, L.-S. Impact of backbone rigidity on the photomechanical response of glassy, azobenzene-functionalized polyimides. Macromolecules 2014, 47, 659–667.
- Wie, J.J.; Wang, D.H.; Lee, K.M.; Tan, L.-S.; White, T.J. Molecular engineering of azobenzene-functionalized polyimides to enhance both photomechanical work and motion. Chem. Mater. 2014, 26, 5223–5230.
- Baczkowski, M.L.; Wang, D.H.; Lee, D.H.; Lee, K.M.; Smith, M.L.; White, T.J.; Tan, L.-S. Photomechanical deformation of azobenzene-functionalized polyimides synthesized with bulky substituents. ACS Macro Lett. 2017, 6, 1432–1437.
- Skandani, A.A.; Chatterjee, S.; Wang, D.H.; Tan, L.-S.; White, T.J.; Shankar, M.R.; Smith, M.L. Relaxation dynamics and strain persistency of azobenzene-functionalized polymers and actuators. Macromol. Mater. Eng. 2017, 302, 1700256.
- Li, Y.; He, Y.; Tong, X.; Wang, X.G. Photoinduced deformation of amphiphilic azo polymer colloidal spheres. J. Am. Chem. Soc. 2005, 127, 2402–2403.
- Li, Y.B.; He, Y.N.; Tong, X.L.; Wang, X.G. Stretching effect of linearly polarized Ar+ laser single-beam on azo polymer colloidal spheres. Langmuir 2006, 22, 2288–2291.
- Liu, J.P.; He, Y.N.; Wang, X.G. Azo polymer colloidal spheres containing different amounts of functional groups and their photoinduced deformation behavior. Langmuir 2008, 24, 678–682.
- Liu, J.P.; He, Y.N.; Wang, X.G. Size-dependent light-driven effect observed for azo polymer colloidal spheres with different average diameters. Langmuir 2009, 25, 5974–5979.
- Liu, J.P.; He, Y.N.; Wang, X.G. Influence of chromophoric electronwithdrawing groups on photoinduced deformation of azo polymer colloids. Polymer 2010, 51, 2879–2886.
- Wang, D.R.; Ye, G.; Wang, X.G. Synthesis of aminoazobenzene-containing diblock copolymer and photoinduced deformation behavior of its micelle-like aggregates. Macromol. Rapid Commun. 2007, 28, 2237–2243.
- Wang, D.R.; Ye, G.; Zhu, Y.; Wang, X.G. Photoinduced mass-migration behavior of two amphiphilic side-chain azo diblock copolymers with different length flexible spacers. Macromolecules 2009, 42, 2651–2657.
- Wang, D.R.; Liu, J.P.; Ye, G.; Wang, X.G. Amphiphilic block copolymers bearing strong push-pull azo chromophores: Synthesis, micelle formation and photoinduced shape deformation. Polymer 2009, 50, 418–427.
- Wang, D.; Wang, X.G. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Prog. Polym. Sci. 2013, 38, 271–301.
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097.
- Broer, D.J.; Bastiaansen, C.M.W.; Debije, M.G.; Schenning, A.P.H.J. Functional organic materials based on polymerized liquid-crystal monomers: Supramolecular hydrogen-bonded systems. Angew. Chem. Int. Ed. 2012, 51, 7102–7109.
- Vapaavuori, J.; Bazuin, C.G.; Priimagi, A. Supramolecular design principles for efficient photoresponsive polymer-azobenzene complexes. J. Mater. Chem. C 2018, 6, 2168–2188.
- Yu, H.-T.; Tang, J.-W.; Feng, Y.-Y.; Feng, W. Structural design and application of azo-based supramolecular polymer systems. Chin. J. Polym. Sci. 2019, 37, 1183–1199.
- Yu, Y.; Maeda, T.; Mamiya, J.; Ikeda, T. Photomechanical effects of ferroelectric liquid-crystalline elastomers containing azobenzene chromophores. Angew. Chem. Int. Ed. 2007, 46, 881–883.
- Yoshino, T.; Kondo, M.; Mamiya, J.; Kinoshita, M.; Yu, Y.; Ikeda, T. Three-dimensional photomobility of crosslinked azobenzene liquid-crystalline polymer fibers. Adv. Mater. 2010, 22, 1361–1363.
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93.
- Chakma, P.; Konkolewicz, D. Dynamic covalent bonds in polymeric materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695.
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32, 1906876.
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233.
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968.
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667.
