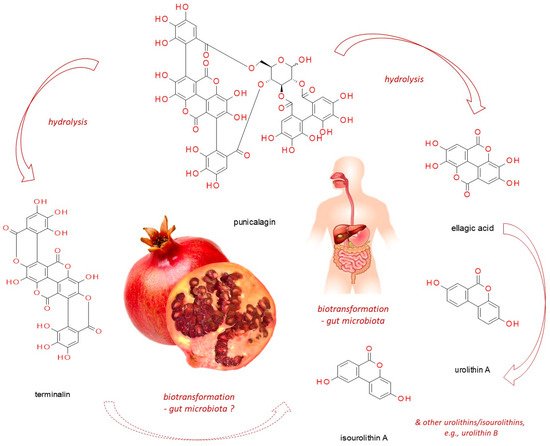
Punicalagin, present in pomegranate, myrobalan, yellow wood, tropical almond, and pink rock-rose, belongs to ellagitannins - a subgroup of hydrolyzable tannins. In vitro studies, based on cell line experiments, have demonstrated punicalagin anti-cancer actions on human cervical, ovarian, breast, lung, thyroid, colorectal, central nervous system, bone, as well as other cancer types. Punicalagin seems to work through a redirection of signal-transduction pathways from survival and proliferation into cell-cycle arrest, apoptosis, senescence or autophagy (thus compromising neoplastic progression). However, since it readily undergoes hydrolysis releasing e.g. ellagic acid, these might be its products which are responsible for the observed effects. Moreover, when practical application of punicalagin in chemoprevention is addressed, its biotransformation in the human organism should be considered (including both host and microbiome enzymatic impact). Therefore, however promising punicalagin antineoplastic properties seem to be, in vivo-based research should be conducted before translating the results obtained from in vitro studies into practice.
- punicalagin
- pomegranate
- ellagitannins
- breast cancer
- cervical cancer
- ovarian cancer
- colorectal cancer
- thyroid cancer
- apoptosis
- autophagy
1. Pomegranate against Pathologies Development
2. Anti-Cancer Activities of Punicalagin (Pug)
2.1. Punicalagin against Cervical Cancer
| Cancer Type | Experimental Model | Up-Regulation ↑ | Down-Regulation ↓ | Final Effect | Ref |
|---|---|---|---|---|---|
| Cervical carcinoma | ME-180 cervical cancer cell line | Bax; Casp-3 and 9; p53; cytosolic NF-κB-p65 | Bcl-2; nuclear NF-κB-p65 | Inhibition of cell proliferation and induction of apoptosis via suppressing NF-κB signaling | [19] |
| Cervix epitheloid carcinoma | HeLa cervical cancer cell line | Bax; TIMP2/3 |
Bcl-2; β-catenin, C-myc, cyclin D1; MMP2/9 |
Inhibition of cell proliferation and migration Cell cycle arrest at G1 phase Suppression of β-catenin pathway Induction of apoptosis |
[20] |
| Breast adenocarcinoma | MCF-7 and MDA-MB-231 cell lines | E-cadherin | GOLPH3; MMP2/9; N-cadherin |
Suppression of cell viability, EMT and migration via the regulation of GOLPH3 | [26] |
| Ovarian cancer | A2780 ovarian cancer cells | Bax; TIMP2/3 |
Bcl-2; β-catenin, cyclin D1, survivin; MMP2/9 |
Inhibition of cell viability and migration Cell cycle arrest at G1 phase Suppression of β-catenin pathway Induction of apoptosis |
[27] |
| Lung carcinoma | Lung cancer A549 cell line | Bax; Casp-3 and 9; cytochrome C; ROS; cytosolic STAT-3 | Bcl-2; Jak-1; nuclear STAT-3 | Inhibition of cell proliferation and induction of apoptosis via suppressing STAT-3 activation | [28] |
| Lung carcinoma | Lung cancer A549 cell line | Casp-3, 8, and 9; PARP-1; mitochondrial ROS | Cytosolic ROS | Induction of apoptosis; cell cycle arrest at G1/S | [29] |
| Osteosarcoma | Cell lines U2OS, SaOS2 | - | Phosphorylated IκBα; nuclear NF-κB-p65; IL-6; IL-8 | Inhibition of cell proliferation and induction of apoptosis possibly via suppressing NF-κB signaling Reduction of invasion potential |
[30] |
| Colorectal carcinoma | Cell line HCT116 | Cytochrome C | Annexin A1; caspases 3/7, 8 and 9 | Induction of cell death via apoptosis and autophagy | [31] |
| Colon adenocarcinoma | Cell line HT-29 | - | COX-2 | Suppression of inflammatory cell signaling | [32] |
| Colon adenocarcinoma | Cell line Caco-2 | Casp-3 and 9; Cytochrome C; Cyclin E | Bcl-XL; Cyclin A and B1 | Cell cycle arrest at S phase; induction of apoptosis via the intrinsic-mitochondrial pathway stimulation | [33] |
| Papillary thyroid carcinoma | Cell line BCPAP | LC3-II conversion, beclin-1; phosphorylated ERK 1/2 and p38 | p62; phosphorylated p70, S6, and 4E-BP1 | Induction of apoptosis-independent cell death via autophagy through the activation of MAPK and inhibition of mTOR signaling | [34] |
| Papillary thyroid carcinoma | Cell line BCPAP | p-H2A.X; p-ATM | - | Induction of cells death via the ATM-mediated DNA damage response | [35] |
| Papillary thyroid carcinoma | Cell line BCPAP | SA-beta-Gal; cyclin-dependent kinase inhibitor p21; IκBα; nuclear NF-κB-p65; IL-6; IL-1β | - | Induction of senescent growth arrest and senescence-associated secretory phenotype (SASP) through the activation of NF-κB | [36] |
| Glioblastoma astrocytoma | Cell line U87MG | Casp-3 and 9; PARP; Cyclin E; LC3-II cleavage, AMPK-P, p27-P | Bcl-2; Cyclin A and B | Cell cycle arrest at G2/M phase; induction of cell death via apoptosis and autophagy | [37] |
Abbreviations: AMPK-P = phosphorylated AMPK (AMP activated kinase); EMT = epithelial to mesenchymal transition; GOLPH3 = golgi phosphoprotein 3; LC3-II = microtubule-associated protein light chain 3 II; MMP2/9 = matrix metalloproteinases 2 and 9; PARP(−1) = poly(ADP-ribose) polymerase (1); p-H2A.X = phospohorylated histone 2A.X; p-ATM = phospohorylated ATM (ataxia telangiectasia mutated); p27-P = phosphorylated p27; SA-beta-Gal = senescence-associated beta-galactosidase; TIMP2/3 = tissue inhibitors of metalloproteinases 2 and 3.
2.2. Punicalagin against Ovarian Cancer
2.3. Punicalagin against Breast Cancer
2.4. Punicalagin against Colorectal Cancer
2.5. Punicalagin against Thyroid Cancer
2.6. Punicalagin against Lung Cancer
2.7. Punicalagin against Osteosarcoma
2.8. Punicalagin against Glioma
This entry is adapted from the peer-reviewed paper 10.3390/nu13082733
References
- Pham, N.M.; Do, V.V.; Lee, A.H. Polyphenol-rich foods and risk of gestational diabetes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2019, 73, 647–656.
- Palma-Duran, S.A.; Vlassopoulos, A.; Lean, M.; Govan, L.; Combet, E. Nutritional intervention and impact of polyphenol on glycohemoglobin (HbA1c) in non-diabetic and type 2 diabetic subjects: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 975–986.
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Portillo, M.P.; Moreno, J.J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452.
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458.
- Gu, H.F.; Mao, X.Y.; Du, M. Prevention of breast cancer by dietary polyphenols-role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2020, 60, 810–825.
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-Cancer Effects of Green Tea Polyphenols against Prostate Cancer. Molecules 2019, 24, 193.
- Ge, S.; Duo, L.; Wang, J.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877.
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999.
- Satomi, H.; Umemura, K.; Ueno, A.; Hatano, T.; Okuda, T.; Noro, T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol. Pharm. Bull. 1993, 16, 787–790.
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-álvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654.
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for prevention and treatment of cancer: An update. Molecules 2017, 22, 177.
- Danesi, F.; Ferguson, L.R. Could pomegranate juice help in the control of inflammatory diseases? Nutrients 2017, 9, 958.
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64.
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106.
- Fecka, I.; Włodarczyk, M.; Starzec, A. Isolation and structure elucidation of cistusin: A new ellagitannin from Cistus × incanus L. leaves. Ind. Crops Prod. 2020.
- Oelrichs, P.B.; Pearce, C.M.; Zhu, J.; Filippich, L.J. Isolation and structure determination of terminalin A toxic condensed tannin from Terminalia oblongata. Nat. Toxins 1994, 2, 144–150.
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and ellagic acid demonstrate anti-mutagenic activity and inhibition of benzo[a]pyrene induced DNA adducts. Biomed. Res. Int. 2014, 2014, 467465.
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367.
- Zhang, L.; Chinnathambi, A.; Alharbi, S.A.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, G. Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-kB signaling pathway. Saudi J. Biol. Sci. 2020, 27, 1100–1106.
- Tang, J.; Li, B.; Hong, S.; Liu, C.; Min, J.; Hu, M.; Li, Y.; Liu, Y.; Hong, L. Punicalagin suppresses the proliferation and invasion of cervical cancer cells through inhibition of the β-catenin pathway. Mol. Med. Rep. 2017, 16, 1439–1444.
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150.
- Lalle, G.; Twardowski, J.; Grinberg-Bleyer, Y. NF-κB in Cancer Immunity: Friend or Foe? Cells 2021, 10, 355.
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82.
- Tegowski, M.; Baldwin, A. Noncanonical NF-κB in Cancer. Biomedicines 2018, 6, 66.
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800.
- Pan, L.; Duan, Y.; Ma, F.; Lou, L. Punicalagin inhibits the viability, migration, invasion, and EMT by regulating GOLPH3 in breast cancer cells. J. Recept. Signal. Transduct. 2020, 40, 173–180.
- Tang, J.M.; Min, J.; Li, B.S.; Hong, S.S.; Liu, C.; Hu, M.; Li, Y.; Yang, J.; Hong, L. Therapeutic Effects of Punicalagin Against Ovarian Carcinoma Cells in Association with β-Catenin Signaling Inhibition. Int. J. Gynecol. Cancer 2016, 26, 1557–1563.
- Fang, L.; Wang, H.; Zhang, J.; Fang, X. Punicalagin induces ROS-mediated apoptotic cell death through inhibiting STAT3 translocation in lung cancer A549 cells. J. Biochem. Mol. Toxicol. 2021, 35, 1–10.
- Berköz, M.; Krośniak, M. Punicalagin induces apoptosis in a549 cell line through mitochondria-mediated pathway. Gen. Physiol. Biophys. 2020, 39, 557–567.
- Huang, T.; Zhang, X.; Wang, H. Punicalagin inhibited proliferation, invasion and angiogenesis of osteosarcoma through suppression of NF-κB signaling. Mol. Med. Rep. 2020, 22, 2386–2394.
- Ganesan, T.; Sinniah, A.; Chik, Z.; Alshawsh, M.A. Punicalagin regulates apoptosis-autophagy switch via modulation of annexin a1 in colorectal cancer. Nutrients 2020, 12, 2430.
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985.
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625.
- Cheng, X.; Gao, Y.; Yao, X.; Yu, H.; Bao, J.; Guan, H.; Sun, Y.; Zhang, L. Punicalagin induces apoptosis-independent autophagic cell death in human papillary thyroid carcinoma BCPAP cells. RSC Adv. 2016, 6, 68485–68493.
- Yao, X.; Cheng, X.; Zhang, L.; Yu, H.; Bao, J.; Guan, H.; Lu, R. Punicalagin from pomegranate promotes human papillary thyroid carcinoma BCPAP cell death by triggering ATM-mediated DNA damage response. Nutr. Res. 2017, 47, 63–71.
- Cheng, X.; Yao, X.; Xu, S.; Pan, J.; Yu, H.; Bao, J.; Guan, H.; Lu, R.; Zhang, L. Punicalagin induces senescent growth arrest in human papillary thyroid carcinoma BCPAP cells via NF-κB signaling pathway. Biomed. Pharmacother. 2018, 103, 490–498.
- Wang, S.G.; Huang, M.H.; Li, J.H.; Lai, F.I.; Lee, H.M.; Hsu, Y.N. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol. Sin. 2013, 34, 1411–1419.
- Sechi, S.; Frappaolo, A.; Karimpour-Ghahnavieh, A.; Piergentili, R.; Giansanti, M.G. Onco-genic roles of GOLPH3 in the physiopathology of cancer. Int. J. Mol. Sci. 2020, 21, 933.
- Zeng, Z.; Lin, H.; Zhao, X.; Liu, G.; Wang, X.; Xu, R.; Chen, K.; Li, J.; Song, L. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin. Cancer Res. 2012, 18, 4059–4069.
- Tokuda, E.; Itoh, T.; Hasegawa, J.; Ijuin, T.; Takeuchi, Y.; Irino, Y.; Fukumoto, M.; Takenawa, T. Phosphatidylinositol 4-phosphate in the Golgi apparatus regulates cell-cell adhesion and invasive cell migration in human breast cancer. Cancer Res. 2014, 74, 3054–3066.
- Tang, S.; Pan, H.; Wei, W.; Yang, H.; Liu, J.; Yang, R. GOLPH3: A novel biomarker that correlates with poor survival and resistance to chemotherapy in breast cancer. Oncotarget 2017, 8, 105155–105169.
- Fu, Z.; Zhang, S.; Wang, B.; Huang, W.; Zheng, L.; Cheng, A. Annexin A1: A double-edged sword as novel cancer biomarker. Clin. Chim. Acta 2020, 504, 36–42.
- Patton, K.T.; Chen, H.M.; Joseph, L.; Yang, X.J. Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology 2005, 47, 597–601.
- Moghanibashi, M.; Jazii, F.R.; Soheili, Z.S.; Zare, M.; Karkhane, A.; Parivar, K.; Mohamadynejad, P. Proteomics of a new esophageal cancer cell line established from Persian patient. Gene 2012, 500, 124–133.
- Suo, A.; Zhang, M.; Yao, Y.; Zhang, L.; Huang, C.; Nan, K.; Zhang, W. Proteome analysis of the effects of sorafenib on human hepatocellular carcinoma cell line HepG2. Med. Oncol. 2012, 29, 1827–1836.
- Roth, U.; Razawi, H.; Hommer, J.; Engelmann, K.; Schwientek, T.; Uller, S.M.; Baldus, S.E.; Patsos, G.; Corfield, A.P.; Paraskeva, C.; et al. Differential expression proteomics of human colorectal cancer based on a syngeneic cellular model for the progression of adenoma to carcinoma. Proteomics 2010, 10, 194–202.
- Lecona, E.; Barrasa, J.I.; Olmo, N.; Llorente, B.; Turnay, J.; Lizarbe, M.A. Upregulation of Annexin A1 Expression by Butyrate in Human Colon Adenocarcinoma Cells: Role of p53, NF-Y, and p38 Mitogen-Activated Protein Kinase. Mol. Cell. Biol. 2008, 28, 4665–4674.
- Nielsen, M.; Kæstel, C.G.; Eriksen, K.W.; Woetmann, A.; Stokkedal, T.; Kaltoft, K.; Geisler, C.; Röpke, C.; ØDum, N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia 1999, 13, 735–738.
