Neurogenic inflammation in migraine is mainly characterized by the release of neuropeptides such as CGRP and substance P from the trigeminal nerve, leading to arterial vasodilation, plasma protein extravasation, and mast cell degranulation. The involvement of these neuropeptides in migraine is evident, and pro-inflammatory cytokines or chemokines may be involved in this series of reactions.
- blood-brain barrier
- migraine
- neuroinflammation
- IL-1β
- chemokine
- anakinra
1. Introduction
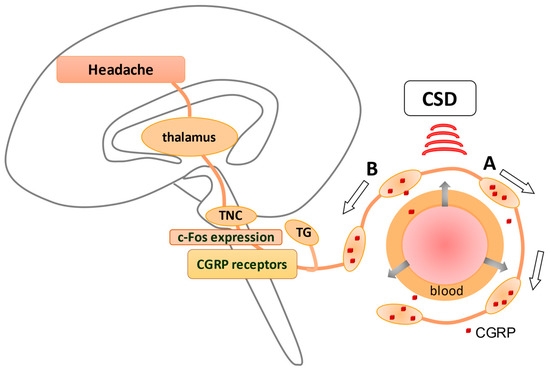
Cortical spreading depression (CSD), relevant to the development of migraine aura, has been shown to activate the trigemino–vascular system (TVS). Anti-temporal conduction of trigeminal ganglion neurons leads to the release of neuropeptides, including calcitonin gene-related peptide (CGRP), from their nerve terminals, leading to vasodilation and plasma extravasation. The dura is densely innervated by sensory nerve fibers that contain these neuropeptides originating from the trigeminal ganglion (TG), causing neurogenic inflammation in the dura (A). In contrast, direct conduction of TG neurons generates a pain sensation via the activation of c-fos in trigeminal nucleus caudalis (TNC) (B), which is eventually perceived as a headache. CGRP receptors are observed in TGs and TNCs but not in peripheral trigeminal nerve terminals, suggesting that CGRP receptor antagonists may suppress neurons in TGs and TNCs. An explanation of how CSD stimulates TVS is given in Figure 1 .
Neurogenic inflammation in migraine is mainly characterized by the release of neuropeptides such as CGRP and substance P from the trigeminal nerve, leading to arterial vasodilation, plasma protein extravasation, and mast cell degranulation. The involvement of these neuropeptides in migraine is evident [1][2], and pro-inflammatory cytokines or chemokines may be involved in this series of reactions [3][4].
Neuroinflammation is essentially defined as an inflammatory response in the brain and spinal cord, which is modulated by the production of cytokines, chemokines, reactive oxygen species (ROS), and secondary messengers such as nitric oxide (NO) and prostaglandins [5][6]. They are mainly produced by activated microglia and astrocytes [5][6], and neurovascular units comprising neurons, pericytes, and endothelial cells [7][8]. Neuroinflammation is closely related to central nervous system (CNS) diseases, such as multiple sclerosis and epilepsy, and the regulation of cytokines has revealed new therapeutic aspects.
This review explores the current literature on neuroinflammation. It targets neuroinflammation from the perspective of cytokines in the pathogenesis of migraine and aims to identify new directions for research on therapeutic interventions for migraine targeting neuroinflammation.
2. Clinical Evidence of Neuroinflammation
During migraine attacks (ictal period), interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor-α (TNF-α) are increased [9][10][11][12][13][14]. Pediatric patients without aura showed higher levels of IL-1β than those with aura [11]. However, some reports have shown no significant difference in TNF-α and IL-1β levels between attacks and attack-free periods [15][16]. Decreased levels of the anti-inflammatory cytokines IL-4 and IL-5 were reported during the attacks [12][17]. Increased levels of IL-10 (an anti-inflammatory cytokine) during attacks, compared with the interictal period [10][18][19], and a decrease in IL-10 by sumatriptan (one of the triptans) were found [19].
During headache-free periods (interictal period), increased pro-inflammatory levels of IL-1β, IL-6, TNF-α, and chemokines such as IL-8, macrophage inflammatory protein-1α (MIP-1α), and C–C chemokine ligand 5 (CCL5) were observed in patients [18][20][21][22][23]. Conversely, IL-10 levels were found to be similar [9] or decreased [19][20][21] when compared with healthy individuals.
While there is some directionality, such as an increase in pro-inflammatory cytokines during both migraine attacks and interictal periods, no definite consensus has been reached. The duration of headache attacks varies widely, which may explain the inconsistent results in terms of sampling timing. Serial analysis of cytokines from jugular venous blood by Sarchielli et al. yielded consistent and robust data which, quite intriguingly, were completely different from data taken from the periphery [24][25]. Significant increases in TNF-α, IL-6, nuclear factor (NF)-κB, and soluble intercellular adhesion molecule-1 (sICAM-1) were observed in parallel within 2 h of attack onset compared with the time of catheter insertion [25]. CGRP increased significantly after 1 h and IL-8 reached its highest level at 4 h, while the other two chemokines, RANTES (CCL5) and monocyte chemoattractant protein-1 (MCP-1), did not significantly change at any time point [24]. While a slight increase in IL-1β was observed from 1 to 4 h, the levels of IL-1β decreased, reaching values at the end of the attack [12]. The levels of cytokines, selectins, vascular adhesion molecules, and adhesion molecules in the peripheral blood of patients with migraine were found to be unaltered at each time point of the study [24][25]. The results of these investigations are consistent and significant over time, illustrating the difficulty of examining cytokines in patients with migraine. Carotid artery blood may provide clearer information than peripheral blood regarding biochemical and neurotransmitter changes in the cerebral circulation, and may be more characteristic of migraine attacks.
Despite the normal serum TNF-α levels in patients with chronic migraine (CM), elevated TNF-α levels in the CSF have been identified [26]. Although IL-1 receptor antagonist (IL-1RA), MCP-1, and transforming growth factor-β1 were significantly increased in the CSF of patients with episodic tension-type headache and in migraine patients with and without aura compared to those without pain, the increases were not sufficiently different between these headache types [27]. A comparison of CSF and plasma or serum cytokine levels would provide some insight into the origin of cytokines, although the assessment of CSF cytokines in patients with migraine is limited. Due to ethical issues, it may be difficult to examine CSF in patients with migraine for the foreseeable future, hence the value of studying available reports.
3. Presence of Blood-Brain Barrier (BBB) Permutability in Migraine
The presence or absence of BBB impairment associated with neuroinflammation is important when considering therapeutic strategies. Studying a drug’s permeability from the periphery to the brain, and its potential to infiltrate cells such as monocytes that introduce cytokines into the brain, will lead to new therapeutic strategies in the future.
Experimental studies of rat models resembling primary headache have shown that CSD modulates the permeability of the BBB by activating matrix metalloproteinase-9 (MMP-9) from 3 h after CSD induction, peaking at 24 h [28]. A recent study in awake rats also found that cortical BBB leakage began 0.5 h after CSD induction and resolved within 6 h, without altering the tight junction proteins occludin or claudin-5 [29]. CSD-induced BBB opening to water and large molecules is mediated by increased endothelial transcytosis. These phenomena are dependent on caveolin-1 and rho-kinase 2; in contrast, endothelial tight junctions, pericytes, and basement membranes are maintained after CSD [30]. This change in BBB permeability is not destructive and may be a transient alteration associated with an inflammatory response. It may also directly affect the access of agents to centrally located targets during migraine attacks [31]. Indeed, increased uptake of sumatriptan into the brain was detected, in association with transient BBB permeability, in a KCl-induced model of episodic headache [29]. Previous reports suggest that dural inflammation induced by trigeminal ganglion (TG) stimulation does not affect the integrity of the BBB [32].
Previous clinical studies have found no association between primary headache and BBB opening [33]. Elevations of MMP-9 [34] and ICAM-1 [13], which are considered typical markers suggestive of BBB disorders, are observed in patients with migraine. An MMP-9 haplotype was reported to affect circulating MMP-9 levels in women with migraine [35].
One study reported a significant elevation of endothelial cell-specific molecule-1 (ESM-1) and claudin-5 in the migraine attack group [14]. ESM-1 is expressed in the vascular endothelium and is regulated by a number of cytokines, including IL-1β and TNF-α, and growth factors. Claudin-5 regulates BBB permeability and is important in sustaining the integrity of cerebrovascular endothelial cells [36][37]. A positive correlation was also found between Visual Analog Scale scores and the levels of ESM-1 and claudin-5 [14]. In addition to the association of BBB disorders with the pathogenesis of migraine, the results suggest a link between the clinical severity of migraine and BBB disorders.
4. Participation of Pericytes in Migraine
Pericytes are a neurovascular unit component of the BBB and play an important role in the integrity of the BBB. Pericyte degeneration and/or dysfunction contribute to the loss of BBB integrity, which is an early hallmark of several neurodegenerative and inflammatory conditions [7][38][39].
Capillary pericytes are also shown to play an active role in the regulation of cortical vasculature during and after CSD [40]. In fact, prolonged vasoconstriction caused by CSD was revealed to be strongest in primary capillaries, where pericytes have a sustained increase in calcium levels. Somatosensory stimulation after CSD causes no further changes in the diameter of capillaries or calcium in pericytes, suggesting that pericytes play a critical role in long-term oligemia after CSD [40]. Current research has demonstrated that brain pericytes respond to inflammatory signals, including IL-1β and TNF-α [38][41][42][43][44]. Pericytes may act as sensors for the inflammatory response in the CNS [43][45]. Based on BBB integrity and systemic peripheral inflammation, pericytes are speculated to play a pivotal role in the pathogenesis of epilepsy during neuroinflammation [8]. Structural changes in pericytes associated with epileptic seizures are exacerbated by IL-1β rather than TNF-α [46]. It is uncertain whether migraine, like epilepsy, is associated with BBB disorder and neuroinflammation, and experimental results targeting pericytes have not been obtained. However, structural changes in pericytes and the endothelial cells of microvessels were also observed in patients with familial hemiplegic migraine (FHM) [47]. It is known that both migraine and epilepsy share pathologies and clinical features to a certain extent, through similar underlying pathophysiological mechanisms, and antiepileptics such as valproic acid or topiramate are effective in treating patients with both disorders [48][49]. Therefore, the prevention or treatment of pericyte constriction may become a therapeutic target in migraine [40][50].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168929
References
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314.
- Malhotra, R. Understanding migraine: Potential role of neurogenic inflammation. Ann. Indian Acad. Neurol. 2016, 19, 175–182.
- Gao, Y.J.; Ji, R.R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68.
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490.
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153.
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316.
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215.
- Yamanaka, G.; Takata, F.; Kataoka, Y.; Kanou, K.; Morichi, S.; Dohgu, S.; Kawashima, H. The Neuroinflammatory Role of Pericytes in Epilepsy. Biomedicines 2021, 9, 759.
- Covelli, V.; Munno, I.; Pellegrino, N.M.; Di Venere, A.; Jirillo, E.; Buscaino, G.A. Exaggerated spontaneous release of tumor necrosis factor-alpha/cachectin in patients with migraine without aura. Acta Neurol. 1990, 12, 257–263.
- Perini, F.; D’Andrea, G.; Galloni, E.; Pignatelli, F.; Billo, G.; Alba, S.; Bussone, G.; Toso, V. Plasma Cytokine Levels in Migraineurs and Controls. Headache J. Head Face Pain 2005, 45, 926–931.
- Kacinski, M.; Gergont, A.; Kubik, A.; Steczkowska-Klucznik, M. Proinflammatory cytokines in children with migraine with or without aura. Przegl. Lek. 2005, 62, 1276–1280.
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46, 200–207.
- Wang, F.; He, Q.; Ren, Z.; Li, F.; Chen, W.; Lin, X.; Zhang, H.; Tai, G. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol. Sci. 2015, 36, 535–540.
- Yücel, M.; Kotan, D.; Gurol Çiftçi, G.; Çiftçi, I.H.; Cikriklar, H.I. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 930–936.
- Van Hilten, J.J.; Ferrari, M.D.; Van der Meer, J.W.M.; Gijsman, H.J.; Looij, B.J., Jr. Plasma interleukin-1, tumour necrosis factor and hypothalamic-pituitary-adrenal axis responses during migraine attacks. Cephalalgia 1991, 11, 65–67.
- Tanure, M.T.; Gomez, R.S.; Hurtado, R.C.; Teixeira, A.L.; Domingues, R.B. Increased serum levels of brain-derived neurotropic factor during migraine attacks: A pilot study. J. Headache Pain 2010, 11, 427–430.
- Martelletti, P.; Stirparo, G.; Morrone, S.; Rinaldi, C.; Giacovazzo, M. Inhibition of intercellular adhesion molecule-1 (ICAM-1), soluble ICAM-1 and interleukin-4 by nitric oxide expression in migraine patients. J. Mol. Med. 1997, 75, 448–453.
- Fidan, I.; Yüksel, S.; Ymir, T.; Irkeç, C.; Aksakal, F.N. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J. Neuroimmunol. 2006, 171, 184–188.
- Munno, I.; Marinaro, M.; Bassi, A.; Cassiano, M.A.; Causarano, V.; Centonze, V. Immunological aspects in migraine: Increase of IL-10 plasma levels during attack. Headache 2001, 41, 764–767.
- Boćkowski, L.; Sobaniec, W.; Zelazowska-Rutkowska, B. Proinflammatory plasma cytokines in children with migraine. Pediatr. Neurol. 2009, 41, 17–21.
- Uzar, E.; Evliyaoglu, O.; Yucel, Y.; Ugur Cevik, M.; Acar, A.; Guzel, I.; Islamoglu, Y.; Colpan, L.; Tasdemir, N. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1111–1116.
- Oliveira, A.B.; Bachi, A.L.L.; Ribeiro, R.T.; Mello, M.T.; Tufik, S.; Peres, M.F.P. Unbalanced plasma TNF-α and IL-12/IL-10 profile in women with migraine is associated with psychological and physiological outcomes. J. Neuroimmunol. 2017, 313, 138–144.
- Duarte, H.; Teixeira, A.L.; Rocha, N.P.; Domingues, R.B. Increased interictal serum levels of CXCL8/IL-8 and CCL3/MIP-1α in migraine. Neurol. Sci. 2015, 36, 203–208.
- Sarchielli, P.; Alberti, A.; Vaianella, L.; Pierguidi, L.; Floridi, A.; Mazzotta, G.; Floridi, A.; Gallai, V. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache 2004, 44, 961–968.
- Sarchielli, P.; Floridi, A.; Mancini, M.L.; Rossi, C.; Coppola, F.; Baldi, A.; Pini, L.A.; Calabresi, P. NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia 2006, 26, 1071–1079.
- Rozen, T.; Swidan, S.Z. Elevation of CSF Tumor Necrosis Factor α Levels in New Daily Persistent Headache and Treatment Refractory Chronic Migraine. Headache J. Head Face Pain 2007, 47, 1050–1055.
- Bø, S.H.; Davidsen, E.M.; Gulbrandsen, P.; Dietrichs, E.; Bovim, G.; Stovner, L.J.; White, L.R. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia 2009, 29, 365–372.
- Gursoy-Ozdemir, Y.; Qiu, J.; Matsuoka, N.; Bolay, H.; Bermpohl, D.; Jin, H.; Wang, X.; Rosenberg, G.A.; Lo, E.H.; Moskowitz, M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Investig. 2004, 113, 1447–1455.
- Cottier, K.E.; Galloway, E.A.; Calabrese, E.C.; Tome, M.E.; Liktor-Busa, E.; Kim, J.; Davis, T.P.; Vanderah, T.W.; Largent-Milnes, T.M. Loss of Blood-Brain Barrier Integrity in a KCl-Induced Model of Episodic Headache Enhances CNS Drug Delivery. eNeuro 2018, 5.
- Sadeghian, H.; Lacoste, B.; Qin, T.; Toussay, X.; Rosa, R.; Oka, F.; Chung, D.Y.; Takizawa, T.; Gu, C.; Ayata, C. Spreading depolarizations trigger caveolin-1-dependent endothelial transcytosis. Ann. Neurol. 2018, 84, 409–423.
- Harriott, A.M.; Takizawa, T.; Chung, D.Y.; Chen, S.P. Spreading depression as a preclinical model of migraine. J. Headache Pain 2019, 20, 45.
- Lundblad, C.; Haanes, K.A.; Grände, G.; Edvinsson, L. Experimental inflammation following dural application of complete Freund’s adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J. Headache Pain 2015, 16, 91.
- Edvinsson, L.; Tfelt-Hansen, P. The blood-brain barrier in migraine treatment. Cephalalgia 2008, 28, 1245–1258.
- Imamura, K.; Takeshima, T.; Fusayasu, E.; Nakashima, K. Increased plasma matrix metalloproteinase-9 levels in migraineurs. Headache 2008, 48, 135–139.
- Martins-Oliveira, A.; Gonçalves, F.M.; Speciali, J.G.; Fontana, V.; Izidoro-Toledo, T.C.; Belo, V.A.; Dach, F.; Tanus-Santos, J.E. Specific matrix metalloproteinase 9 (MMP-9) haplotype affect the circulating MMP-9 levels in women with migraine. J. Neuroimmunol. 2012, 252, 89–94.
- Dong, L.; Qiao, H.; Zhang, X.; Zhang, X.; Wang, C.; Wang, L.; Cui, L.; Zhao, J.; Xing, Y.; Li, Y.; et al. Parthenolide is neuroprotective in rat experimental stroke model: Downregulating NF-κB, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediat. Inflamm. 2013, 2013, 370804.
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Lortat-Jacob, H.; Bechard, D.; Lassalle, P.; Delehedde, M. Endocan or endothelial cell specific molecule-1 (ESM-1): A potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta 2006, 1765, 25–37.
- Jansson, D.; Rustenhoven, J.; Feng, S.; Hurley, D.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.; Dragunow, M. A role for human brain pericytes in neuroinflammation. J. Neuroinflamm. 2014, 11, 104.
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78.
- Khennouf, L.; Gesslein, B.; Brazhe, A.; Octeau, J.C.; Kutuzov, N.; Khakh, B.S.; Lauritzen, M. Active role of capillary pericytes during stimulation-induced activity and spreading depolarization. Brain 2018, 141, 2032–2046.
- Kovac, A.; Erickson, M.A.; Banks, W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflammation 2011, 8, 139.
- Alcendor, D.J.; Charest, A.M.; Zhu, W.Q.; Vigil, H.E.; Knobel, S.M. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J. Neuroinflammation 2012, 9, 95.
- Matsumoto, J.; Takata, F.; Machida, T.; Takahashi, H.; Soejima, Y.; Funakoshi, M.; Futagami, K.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Tumor necrosis factor-α-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci. Lett. 2014, 578, 133–138.
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302.
- Matsumoto, J.; Dohgu, S.; Takata, F.; Machida, T.; Bölükbaşi Hatip, F.F.; Hatip-Al-Khatib, I.; Yamauchi, A.; Kataoka, Y. TNF-α-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IκB-NFκB and JAK-STAT3 pathways. Brain Res. 2018, 1692, 34–44.
- Klement, W.; Garbelli, R.; Zub, E.; Rossini, L.; Tassi, L.; Girard, B.; Blaquiere, M.; Bertaso, F.; Perroy, J.; de Bock, F.; et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol. Dis. 2018, 113, 70–81.
- Dziewulska, D.; Kierdaszuk, B. Ultrastructural changes in microvessels in familial hemiplegic migraine with CACNA1A mutation. Clin. Neuropathol. 2018, 37, 283–287.
- Kossoff, E.H.; Andermann, F. Migraine and Epilepsy. Semin. Pediatr. Neurol. 2010, 17, 117–122.
- Mantegazza, M.; Cestèle, S. Pathophysiological mechanisms of migraine and epilepsy: Similarities and differences. Neurosci. Lett. 2018, 667, 92–102.
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018, 136, 507–523.
