2.1. Development of Meristematic and Conductive Tissues in Stem
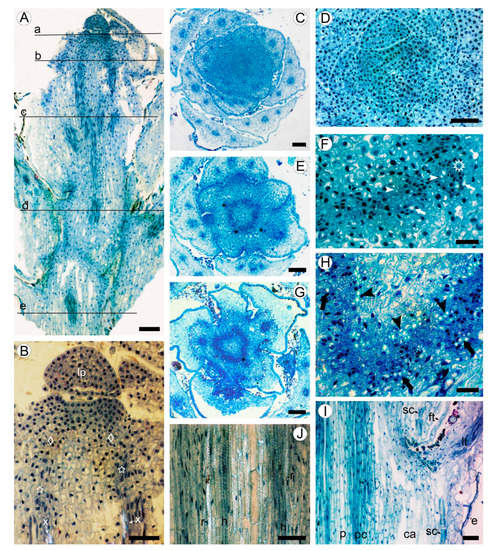
Shoot apical meristem (SAM) is about 200 µm in diameter, surrounded with the leaf primordia and small leaves arranged with the 3/8 phyllotaxis (Figure 1A,B). The procambium is initiated in the peripheral zone of SAM (primary meristem) near the shoot tip. The procambial strands are hardly distinguished, forming nearly continuous ring of four to six cells wide, occasionally interrupted by the offsets of leaf traces (Figure 1A–D). The procambial cells are angular isodiametric (6–9 µm in width) in transverse view, and elongated (26–48 µm in length) on longitudinal sections. Differentiation of the protoxylem and protophloem begins almost immediately with the formation of groups of one to two helical tracheary elements and one to three sieve elements (Figure 1E,F).
Figure 1. Apical meristem and primary tissues in young shoot of Aragoa corrugatifolia, light microscopy (LM). (A) Radial longitudinal section (RLS) of young shoot, approximate positions of the transverse sections (TS) shown on Figure 1C,D (a), Figure 1E,F (b), Figure 1G,H (c), Figure 11I,J (d), and Figure 2A,B (e); (B) tip of young shoot observed in partially polarized light (RLS), shoot apical meristem (SAM) with leaf primordium (lp) and peripheral zone (diamonds), procambium cells (stars), protoxylem elements (x); (C) shoot tip at the SAM level (TS), 3/8 phyllotaxis, leaf primordia with solitary leaf traces, young leaves with three vascular bundles; (D) SAM with leaf primordia (TS); (E) shoot tip just below the SAM level (TS); procambium ring, leaf traces (asterisks); (F) portion of the procambium ring shown on Figure 1E (TS), leaf trace (asterisk), protoxylem (arrowheads), and protophloem (arrows) elements; (G) young shoot in the lower portion of first elongated internode (TS), procambium ring, offset of leaf trace (asterisk). (H) Portion of the procambium ring shown on Figure 1G (TS), radial seriations of procambial cells, elements of primary xylem (arrowheads) and primary phloem (arrows); (I) stem and leaf base at the level of fifth elongated internode (RLS), procambium ring (pc), pith (p), cortical aerenchyma (ca), sclereids at the leaf base (sc), epidermis, leaf trace (lt), filiform trichome (ft); (J) portion of the procambium ring shown in Figure 1I observed in partially polarized light (RLS), tracheary elements with helical (h) and reticulate (r) patterns of secondary cell wall. Scale bars: 100 µm for (A,C,E,G), 50 µm for (B,D,I,J), 20 µm for (F,H).
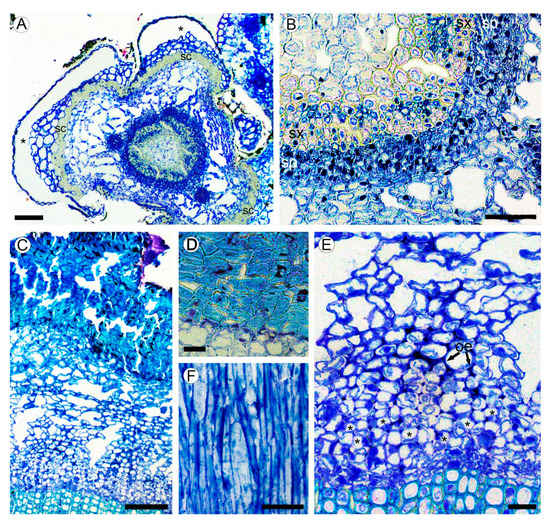
Figure 2. (A,B) Structure of young stem in Aragoa corrugatifolia (LM). (A) Young shoot with prominent leaf bases in the lower portion of fourth elongated internode (TS), vascular cambium with juvenile rings of secondary xylem and secondary phloem, cortical aerenchyma, leaf bases (asterisks) outlined with the bands of sclereids (sc); (B) portion of the ring of secondary conductive tissues shown in Figure 2A (TS), continuous rings of secondary xylem (sx) and secondary phloem (sp). (C–F) Structure of mature bark. (C) Secondary phloem, cortex with aerenchyma, periderm with uniseriate phelloderm, and prominent phellem made of thick-walled cell (TS); (D) Portion of periderm, phellem cells with thick sclerified walls, thin-walled phelloderm cells in one to two layers (TS); (E) portion of secondary phloem and cortical aerenchyma shown in Figure 2C (TS), sieve tubes with companion cells in radial groups (*), obliterated phloem elements (oe), large intercellular spaces in cortex; (F) tangential longitudinal section (TLS) of secondary phloem; strands of phloem axial parenchyma, lack of rays. Scale bars: 100 µm for (A,C), 50 µm for (B,F), 20 µm for (D,E).
At the level of the base of the first elongated internode (ca. 0.4 mm from the apex), the procambium ring widens up to 7–10 cells wide, interrupted only by the gaps at the offsets of leaf traces (Figure 1G,H). The procambial cells in the ring show a tendency for radial seriation; the sites of leaf trace initiation can be distinguished as the clusters of cells divided at various orientations. The elements of primary phloem are in groups of two to four scattered along the outer side of the procambium ring. These tracheary elements are solitary and grouped as two to five cells, found in the inner region of procambium ring, without obvious association with the groups of phloem elements. The proto- and metaxylem consists of tracheary elements with helical and reticulate patterns of secondary cell walls.
In the lower internodes, the vertical length of procambium cells increases due to the shoot growth up to 35–67 µm in the fifth internode (Figure 1I,J). These cells are more tapered than those near the shoot tip. Some cells undergo transverse divisions, forming the strands of two cells. No short procambium cells resembling the ray initials were found. Below the sixth internode, the procambium ring is gradually transformed into the vascular cambium, which is recognized by the appearance of the continuous rings of regularly arranged phloem and xylem elements around the pith made of thin-walled isodiametric parenchyma cells of 15–25 µm, with large intercellular spaces in between. Sclereids with moderately thick cell walls were found in the outermost region of pith (Figure 2A,B).
2.2. Bark Structure
A large portion of the circumference of young stems is occupied by the bases of leaves covered with the leaf abaxial epidermis. The zones of attachment of leaf bases to the stem cortex are outlined with the uniseriate (occasionally biseriate) layers of thick-walled vertically elongated sclereids of 18–34 µm in tangential size and 32–54 µm in vertical size (Figure 1J and Figure 2A). Between the leaf bases, the epidermis on young parts of stems is composed of a single layer of isodiametric cells (15–25 µm in tangential size) with evenly thick walls (3–7 µm thick) covered by a prominent cuticle that is 8–10 µm thick, with dark deposits. The cortex is narrow (10–12 cells in width) and composed of aerenchyma made isodiametric to elongated thin-walled parenchyma cells of 15–35 µm in size, with large intercellular spaces in-between. No crystals were found in the cortical parenchyma cells. Primary phloem fibers were absent (Figure 2A,B). Dilation of the cortical tissue is effected mostly by tangential stretching of parenchyma cells and intercellular spaces.
Mature bark is dark brown, non-peeling, without fissures and scales. The initiation of periderm is in the subepidermal layer of cells. The phellem is composed of 8–15 layers isodiametric to somewhat flattened cells with thick to very-thick sclerified cell wall occasionally with dark deposits. The phelloderm comprises one to two layers of isodiametric, thin-walled cells. No crystalliferous cells were found in periderm. Subsequent periderms were not found (Figure 2C,D).
Sieve tubes are in radial groups of two to seven. Sieve tubes members are 148–236 µm (average 184.2 µm) in length and are 9–18 µm wide. Sieve plates are compounded with two to three sieve areas, located on vertical or slightly oblique cross walls. Axial parenchyma cells are associated with conducting elements that occur as single fusiform cells and in strands of two to three cells. The transition from conducting to nonconducting secondary phloem is gradual, marked with obliterated conductive cells and slightly tangentially stretched axial parenchyma cells (Figure 2E). Secondary phloem rays were not found (Figure 2F).
2.3. Leaf Structure
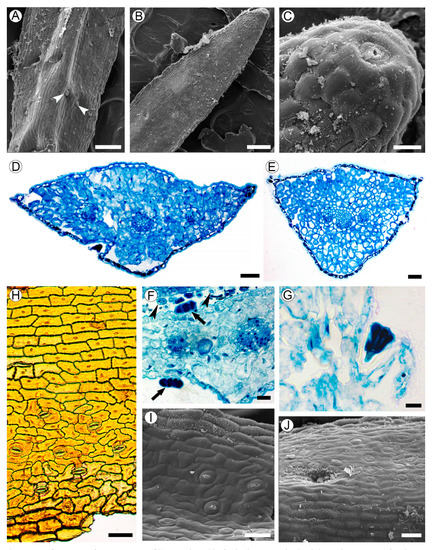
Leaves are linear, 1–2 mm in width, sessile, with convex abaxial side, and flat adaxial surface, and are 200–400 µm thick. The adaxial epidermal cells do not differ from the abaxial ones in their shape and size; these cells are flattened to isodiametric, 18–30 µm in tangential size, with even walls of approximately 1 µm thick (Figure 3A,B). Hydathode pores are present at the leaf tips (Figure 3C).
Figure 3. Leaf structure of Aragoa corrugatifolia. (A) Abaxial leaf side (SEM), sessile glandular trichomes (arrowheads); (B) adaxial leaf side (SEM), hydathode pore on the leaf tip; (C) hydathode pore at the leaf tip shown on Figure 3B (SEM); (D) TS of leaf in its middle portion (LM), epidermis with prominent cuticle, spongy mesophyll, three vascular bundles; (E) TS of leaf in its basal portion (LM), epidermis with prominent cuticle, spongy mesophyll, branch point of vascular bundles; (F) fragment of leaf (TS, LM), sessile glandular trichomes on adaxial and abaxial epidermis (arrows), cross sections of filiform trichomes (arrowheads); (G) stalked glandular trichome on adaxial epidermis (LM); (H) Abaxial epidermis (LM) from midrib without stomata (top) and lateral portions of leaf with anomocytic stomata (bottom). (I) Adaxial leaf side (SEM), anomocytic stomata, epidermal cells with curved anticlinal walls. (J) Midrib region on adaxial leaf side (SEM), epidermal cells with straight anticlinal walls, hollow with glandular trichome. Scale bars: 200 µm for (A,B), 50 µm for (C–E,H–J), 20 µm for (F,G).
Leaf blades are isobilateral (Figure 3D,E). The spongy mesophyll consists of irregularly shaped cells of 11–25 µm in size with large intercellular spaces. The vascular bundles are collateral, sheathed by one or two layers of parenchyma cells without lateral or vertical extensions. Each leaf is supplied by a single trace that is forked into three vascular bundles near the leaf base.
Long (3–4 mm in length) multicellular filiform trichomes are found at the bases of juvenile leaves on their adaxial sides, and usually lack mature leaves (Figure 1I and Figure 3F). Glandular trichomes sessile or on unicellular stalks, with four head cells, are located mostly in hollows sparsely scattered on both leaf sides (Figure 3A,F,G,J).
Anticlinal walls of epidermal cells are mostly straight in the midrib region, or mostly curved outside of it. The outer walls of the epidermal cells are covered by a cuticle of approximately 4–7 µm thick. Leaves are amphistomatic; stomata are more numerous on the abaxial side (48–62 per mm2) than on the adaxial side (39–50 per mm2), lacking in the midrib region. Stomata anomocytic is located in the same plane with epidermal cells (Figure 3H–J).
2.4. Wood Structure
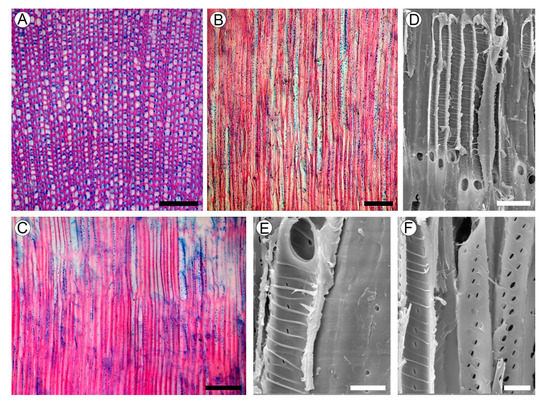
Growth rings absent. Wood is rayless (Figure 4A–C). Vessels are angular; rarely rounded in outline; extremely narrow with a tangential diameter of 9.2–19 µm (average 13.5 µm); and are extremely numerous (1130–1700 per mm2), mostly solitary, and also has multiples. Vessel walls are 1.6–3.4 µm thick. Tyloses were not found. Vessel elements are 102–275 µm long (average 202.6 µm). Perforation plates are simple. Intervessel pits are alternate and minute, 1.8–3.5 µm in vertical size, mostly with rounded margins and slit-like apertures. Helical thickenings throughout the body of the vessel element are common (Figure 4D–F). Ground tissue elements are non-septate libriform fibers of 190–317 µm (average 255.2 µm) in length, mostly with living protoplast and nuclei. Fiber walls are 2.0–5.4 µm thick, with distinctly bordered pits of 2.4–3.5 µm in vertical size, with slit-like apertures in radial and tangential walls. Axial parenchyma was not observed.
Figure 4. Wood structure of Aragoa corrugatifolia. (A) TS of wood (LM), growth rings absent, very narrow vessels, libriform fibers with living protoplasts; axial parenchyma and rays absent; (B) TLS of wood (LM), very narrow vessels, lack of rays; (C) RLS of wood (LM), imperforate elements with numerous pits in radial walls, lack of rays; (D) vessel elements on RLS (SEM), simple perforation plates, helical thickenings on vessel walls; (E) vessel element and fibriform fiber on RLS (SEM), simple perforation plates, helical thickenings on vessel walls, pits on the fiber wall (right); (F) vessel elements on RLS (SEM), helical thickenings on vessel walls; minute intervessel pits. Scale bars: 100 µm for (A–C), 10 µm for (D–F).