Over the years, chiral discrimination of chiral molecules such as amino acids, alcohols, amines, hydroxy-carboxylic acids, etc. has aroused the interest of the scientific community. Thus, numerous studies have reported the possibility to discriminate several organic guests by using both symmetric and asymmetric porphyrin derivatives. Nevertheless, this entry exclusively focuses on chiral porphyrinoids as probes for asymmetric recognition and sensing, illustrating the main aspects concerning the chiral recognition phenomena of a multitude of chiral organic guests through several chiral mono- and bis-porphyrins via different spectroscopic techniques.
- chirality
- chiral recognition
- sensing
- porphyrin
- chiral guest
- circular dichroism
1. Introduction
Two or more molecules, able to non-covalently interact and to display binding-site complementarity, may exhibit molecular recognition [1][2]. In this respect, the chiral recognition is a specific process in which a host molecule selectively binds, through supramolecular forces (e.g., hydrogen bonding, metal coordination, hydrophobic effects, van der Waals forces, π–π and/or electrostatic interactions) to a precise enantiomer of the guest molecule compared with mirror stereoisomer [3][4][5][6][7][8][9][10][11]. Nevertheless, this latter phenomenon represents a key process in a wide range of chemical systems and biological occurrences in the natural world [12][13][14][15][16][17]. In this context, porphyrins, owing to their i) extensive electron conjugation, ii) fascinating spectral and photophysical properties, along with iii) the facile metal coordination and functionalization at meso/β-positions [18][19][20], can be successfully employed as ideal receptors to recognize a large variety of guests [21][22]. For this reason, porphyrinoids and related derivatives find numerous applications in the literature, ranging from energy transfer phenomena and catalysis to oxygen transport and biological and supramolecular self-assembled systems [23][24][25][26][27][28].
Chiral porphyrins and metalloporphyrins are particularly well suited for molecular recognition and optical resolution since they can be useful for the determination of absolute configuration, enantiomeric resolution, and enantiomeric excess of chiral molecules [29][30][31][32][33][34][35][36]. Furthermore, they can mimic biomolecular recognition in biological and biochemical processes [37]. Monomeric porphyrinoids can exhibit two sorts of chirality: i) extrinsic chirality, which occurs when chiral substituents are covalently bonded to the macrocycle ring [38], and ii) intrinsic chirality, in which achiral substituents are arranged along a chiral axis or on a chiral plane to the porphyrin ring [16][39][40]. In addition, chiral dimeric and multimeric porphyrins can be achieved by means of a chiral bridge between chiral or achiral porphyrin rings [18].
Although several studies have reported the opportunity to discriminate numerous guests by using both symmetric and asymmetric porphyrin derivatives [10][41][42][43][44][45][46], this review exclusively focuses on chiral porphyrinoids as probes for recognition and sensing of chiral organic molecules, with particular regard to amino acids, their ester-derivative amines, alcohols, carboxylic acids, and their derivatives, as well as some chiral aromatic compounds—ruling out, however, the corresponding achiral counterparts.
The major advantage of using chiral porphyrins over achiral ones is because of the formation of host–guest diastereomeric complexes that present different physical and chemical properties and can be easily monitored by conventional spectroscopic methods, such as UV/Vis, fluorescence, 1H NMR, and circular dichroism [47]. Noteworthy, among several chiral molecules, as reported in the literature, special attention is given to amino acid guests. In fact, amino acids are fundamental bioactive molecules broadly employed in several chemical and pharmaceutical fields [48]. Additionally and more remarkable, the chiral discrimination of enantiomers from amino acids constitutes a crucial step in better investigating the origin of “homochirality” in biological systems, as well as in developing interesting chiral devices for technological applications [49][50][51]. For instance, in the agri-food field, D- and L-amino acid profiles can give important information to reveal food adulteration [52][53], food quality, and the nutritional value of foods [54]. Nevertheless, chiral discrimination of other chiral organic guests has been taken into account.
2. Chiral Recognition of Amino Acids and Their Esters
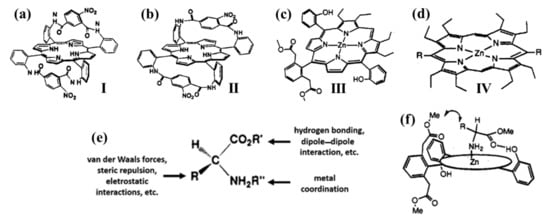
The pioneering research concerning the chiral recognition of amino acids and corresponding methyl ester derivatives was from Professor Y. Kuroda’s group. In fact, between 1993 and 1995, they reported a systematic synthesis of several chiral porphyrinoids to discriminate amino acid methyl esters (Figure 1a–d, I–IV) [55][56][57][58]. In fact, in amino acids, both amino and carboxyl moieties are expected to behave as sites for hydrogen bonding and electrostatic interactions (Figure 1e), whilst the α -R group may form further binding sites (i.e., van der Waals forces, hydrophobic interactions, steric hindrance). Thus, since an effective chiral recognition mechanism demands at least a spatially oriented three (or two)-point interaction, amino acids can appear as suitable guest models for the molecular recognition processes. Nevertheless, if the host system contains a metal ion, a coordination interaction may occur between the guest amino acid and the host system. As a consequence, Kuroda et al., designed Zinc(II)-porphyrin derivatives having two (Figure 1c, III) or three (Figure 1d, IV) recognition elements (i.e., metal coordination, hydrogen bond donor, and hydrogen bond acceptor—or steric repulsion groups) in order to realize a convergent chiral recognition pocket (Figure 1f) [55][56][57][58]. In particular, chiral doubly bridged free-base tetraarylporphyrins (Figure 1a,b I–II), chiral Zinc(II) porphyrins with three arms (5, 10, 15- meso positions, III) and Zinc(II) with two functional arms (5, 15- meso positions, IV) were synthesized as racemic mixtures and resolved by chiral HPLC. As a result, the association constants were determined in halogenated solvents (e.g., chloroform or dichloromethane) by the UV/Vis titration method, proving different binding affinity depending on the sort of amino acid esters used and their own chirality.
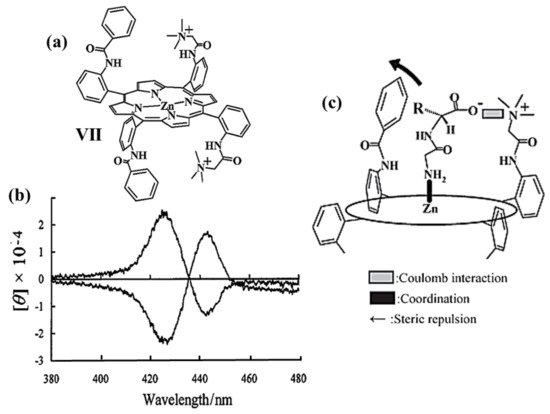
A few years later, in 2004, Imai et al., reported the first study regarding the chiral recognition of amino acids and dipeptides in water at room temperature [59]. They synthesized water-soluble chiral Zn(II)-porphyrin derivative (Figure 2a, VII) with C2 symmetry, prepared as a racemic mixture and resolved by chiral HPLC method. The CD spectra of both enantiomers show specular bisignate couplets in the porphyrin Soret region around 425 nm and 442 nm (Figure 2b). The two Zinc(II)-porphyrin VII enantiomers showed chiral selectivity for L-enantiomers of amino acids and dipeptides by UV/Vis titration method in an aqueous solution. According to Kuroda’s observations, the chiral recognition mechanism suggested by the authors consists of three types of interaction: coulombic, coordination, and steric repulsion, as illustrated in Figure 2c.
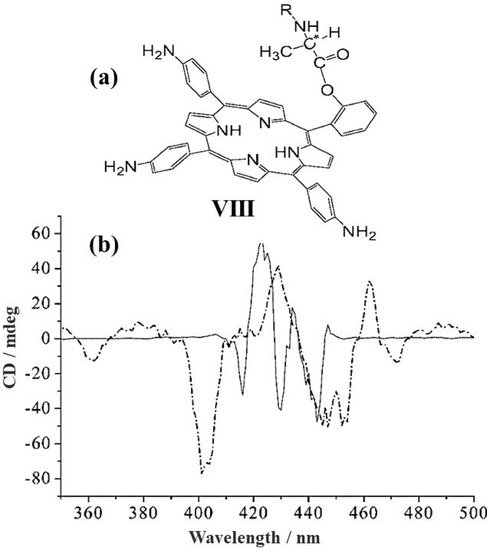
The incorporation of a chiral porphyrin (Figure 3a, VIII) into the mesoporous molecular sieves SBA-15 for chiral recognition of small amino acids was investigated in 2006 by Wang et al. [60]. The resultant material was analyzed by circular dichroism spectroscopy, demonstrating CD signals very different compared with those of the chiral porphyrin alone in solution (Figure 3b). The authors explained such difference in terms of intermolecular interaction of chiral porphyrin VIII with the OH- groups in the channel of SBA-15. Moreover, they demonstrated by UV/Vis and CD spectroscopy that the composite material (SBA-15/porphyrin) has a higher affinity for D-alanine than the L-enantiomer through the mixing in the water of the composite mesoporous materials with the related chiral amino acids at room temperature. Noteworthy, in comparison with L-alanine, the D-enantiomer is more tightly absorbed into the channel and onto the surface of the mesoporous material. This research provides a feasible model to overcome the tedious preparation in organic solvents, along with the weak solubility of some amino acids in an organic environment, facilitating the host–guest separation as well.
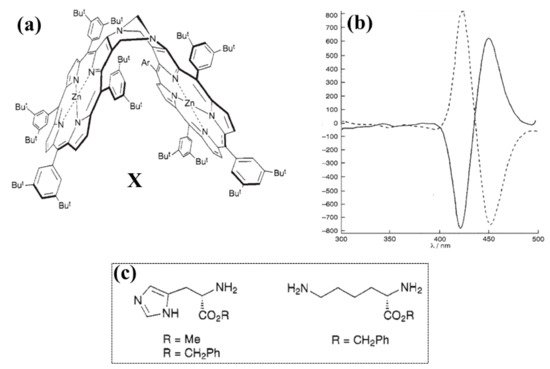
The first work on chiral recognition based on bis-porphyrin systems dates back to 1995, reported by Crossley et al. [61]. They prepared Troger’s base analogue chiral porphyrin dimer (Figure 4a, X) for chiral recognition of methyl and benzyl esters of histidine and lysine amino acids [62]. The bis-porphyrin was prepared as a racemic mixture and resolved by the chiral HPLC technique. The resolved enantiomer pairs have a C2 symmetry, and they display specular CD spectra with a bisignate couplet in the Soret band region (Figure 4b). NMR spectroscopy was used to investigate the affinity of the bis-porphyrin towards chiral amino acid derivates: high affinity was shown for the histidine methyl and benzyl esters (calculated enantiomeric excess up to 80–86%) and for lysine benzyl ester (calculated enantiomeric excess up to 48%) in deuterated chloroform (Figure 4c).
3. Chiral Recognition of Anionic and Neutral Guests
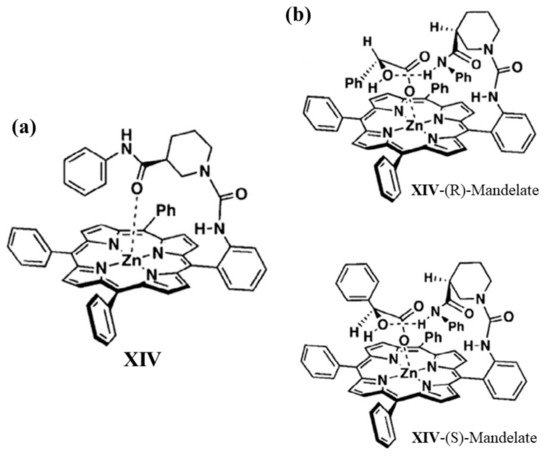
A chiral Zn(II)-porphyrin derivate (Figure 5a, XIV) having N-phenylamide group was reported by Starnes and co-workers in 2012 [63]. Such system was investigated for chiral selectivity towards several anionic guests by using UV/Vis spectrophotometric titration in dichloromethane. In detail, for both enantiomers of N-acetylalanine, N-acetylphenylalanine, and N-acetyltryptophan, chiral zinc-porphyrin exhibits a moderate selectivity. On the other hand, a better selectivity was observed to discriminate (S)- and (R)-mandelate isomers, evidencing a binding ratio (S)-/(R)- isomers around 2. The 1H NMR technique was performed to investigate the mechanism of complexation between chiral porphyrin XIV and mandelate isomers. 1H NMR data suggest the well-known three-point interaction between chiral porphyrin and (S)-mandelate (Figure 5b), whereas for (R)-mandelate a less favorable complex is formed because of steric repulsion between the porphyrin ring and the phenyl group of mandelate (Figure 5b).
In 2005, a chiral bis-porphyrin (Figure 6a, XVI) with a C2 symmetry was reported by Ema et al. [64]. The bis-porphyrin XVI was explored as a receptor and chiral 1H NMR shift agent for chiral diamines, aziridine, and isooxazoline in chloroform (Figure 6b).
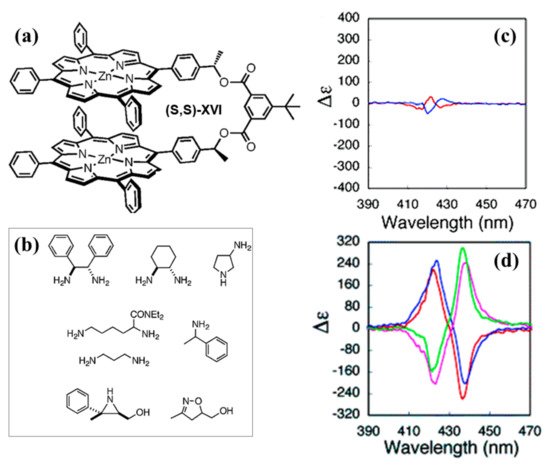
Regarding the spectroscopic sensing, the binding of both chiral and achiral diamines tends to fix the conformation of the two porphyrin units, causing a significant amplification of the CD couplet. However, the effect of enhanced exciton coupling is strictly associated with the chirality and structure of the chiral diamine guests used. Interestingly, the binding with chiral diphenylethylene diamine leads to slight amplification of the CD signal as compared with uncomplexed porphyrin tweezer [64]. Conversely, the binding with chiral diamines having minor steric hindrance, such as cyclohexane diamine (CHDA), closes the two macrocycle chromophores, giving rise to an increased CD (Figure 6d). Noteworthy, in the case of diphenylethylene diamine and cyclohexane diamine, the sign of the CD couplets is governed by the initial chirality of guests used for the complexation (Figure 6 d).
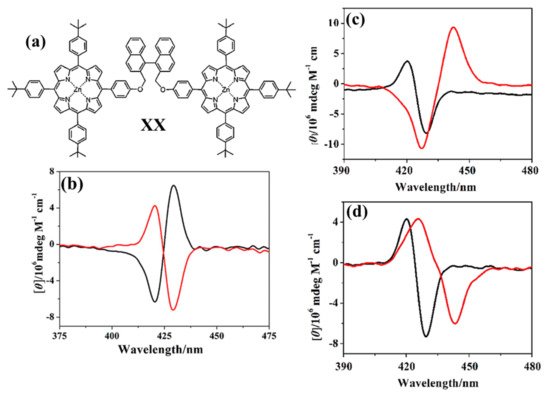
Lu et al., reported in 2017 a chiral binaphthalene-bridged bisporphyrin with C2 symmetry (Figure 7 a, XX) able to recognize chiral diamines in chloroform [65]. The CD spectra of the two enantiomer pairs point out a mirror image with a bisignate couplet in the porphyrin Soret region (Figure 7b). The UV/Vis investigations revealed that both enantiomers showed enantioselectivity towards chiral 1,2 diamines, with the highest ratio of 5.07 observed for (1R, 2R)/(1S, 2S)-diaminecyclohexane. Nevertheless, different and almost specular dichroic spectra were obtained for R-enantiomer of the bis-porphyrin by the addition of (1S, 2S)-(Figure 7c) and (1R, 2R)-diaminecyclohexane (Figure 7d).
4. Conclusions
Chiral recognition is one of the most important issues in the natural world, and it is widespread in biological systems. Indeed, being of particular interest to researchers, such a phenomenon has been extensively studied over the past few decades. The basis of the chiral recognition mechanism is the formation of diastereoisomeric complexes between the enantiomers and a pre-organized chiral selector with a minimum of three-points interaction for the enantiomeric recognition [66][67][68]. Porphyrinoids and bis-porphyrinoids, owing to their versatile synthesis capacity, can be constructed appropriately as hosts for molecular recognition of a specific guest. In this review, several chiral guests were reported, especially amino acids and their derivates, for their biological relevance and analogous molecules, such as amines and diamines. However, most of the studies perform the chiral recognition phenomena in organic solvents (e.g., dichloromethane or chloroform), which do not hinder the supramolecular interactions between the hosts and guest molecules. Thus, the concepts gathered in this review may encourage the design of chiral porphyrinoid hosts able to enantio-recognize chiral molecules in water so as to mimic the biological aqueous environment.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors9080204
References
- Baron, R.; McCammon, J.A. Molecular recognition and ligand association. Annu. Rev. Phys. Chem. 2013, 64, 151–175.
- Persch, E.; Dumele, O.; Diederich, F. Molecular recognition in chemical and biological systems. Angew. Chem. Int. Ed. 2015, 54, 3290–3327.
- Arnaboldi, S.; Benincori, T.; Penoni, A.; Vaghi, L.; Cirilli, R.; Abbate, S.; Longhi, G.; Mazzeo, G.; Grecchi, S.; Panigati, M.; et al. Highly enantioselective “inherently chiral” electroactive materials based on a 2,2′-biindole atropisomeric scaffold. Chem. Sci. 2019, 10, 2708–2717.
- Ousaka, N.; Yamamoto, S.; Iida, H.; Iwata, T.; Ito, S.; Hijikata, Y.; Irle, S.; Yashima, E. Water-mediated deracemization of a bisporphyrin helicate assisted by diastereoselective encapsulation of chiral guests. Nat. Commun. 2019, 10, 1–11.
- Baars, M.W.P.L.; Meijer, E.W. Host-Guest Chemistry of Dendritic Molecules. In Dendrimers II. Topics in Current Chemistry; Vögtle, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 210, pp. 131–182.
- Gattuso, G.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Pisagatti, I. Recognition in water of bioactive substrates by a sulphonato p-tert-butylcalix[5]arene. Supramol. Chem. 2014, 26, 597–600.
- Rekharsky, M.V.; Yamamura, H.; Inoue, C.; Kawai, M.; Osaka, I.; Arakawa, R.; Shiba, K.; Sato, A.; Young, H.K.; Selvapalam, N.; et al. Chiral recognition in cucurbituril cavities. J. Am. Chem. Soc. 2006, 128, 14871–14880.
- Corradini, R.; Sforza, S.; Tedeschi, T.; Marchelli, R. Chirality as a tool in nucleic acid recognition: Principles and relevance in biotechnology and in medicinal chemistry. Chirality 2007, 19, 269–294.
- Zhang, L.; Jin, Q.; Liu, M. Enantioselective Recognition by Chiral Supramolecular Gels. Chem. Asian J. 2016, 11, 2642–2649.
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523.
- Gaeta, M.; Rodolico, E.; Fragalà, M.E.; Pappalardo, A.; Pisagatti, I.; Gattuso, G.; Notti, A.; Parisi, M.F.; Purrello, R.; D’Urso, A. Self-assembly of discrete porphyrin/calix[4]tube complexes promoted by potassium ion encapsulation. Molecules 2021, 26, 704.
- Ceccacci, F.; Mancini, G.; Sferrazza, A.; Villani, C. pH variation as the switch for chiral recognition in a biomembrane model. J. Am. Chem. Soc. 2005, 127, 13762–13763.
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and Amplification of Chirality by Helical Polymers. Chem. Eur. J. 2004, 10, 42–51.
- Guo, D.S.; Wang, K.; Liu, Y. Selective binding behaviors of p-sulfonatocalixarenes in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 1–21.
- Randazzo, R.; Gaeta, M.; Gangemi, C.M.A.; Fragalà, M.E.; Purrello, R.; D’Urso, A. Chiral recognition of L- and D-amino acid by porphyrin supramolecular aggregates. Molecules 2019, 24, 84.
- Lintuluoto, J.M.; Borovkov, V.V.; Inoue, Y. Direct determination of absolute configuration of monoalcohols by bis (magnesium porphyrin). J. Am. Chem. Soc. 2002, 124, 13676–13677.
- De Luca, G.; Romeo, A.; Scolaro, L.M.; Pasternack, R.F. Conformations of a model protein revealed by an aggregating CuII porphyrin: Sensing the difference. Chem. Commun. 2010, 46, 389–391.
- Yang, L.; Zhou, Y.; Zhu, M.; Zhao, L.; Wei, L.; Bian, Y. Stereochemistry and solid-state structure of an intrinsically chiral meso-patterned porphyrin: Case study by NMR and single-crystal X-ray diffraction analysis. J. Org. Chem. 2013, 78, 9949–9955.
- Gaeta, M.; Randazzo, R.; Cristaldi, D.A.; D’Urso, A.; Purrello, R.; Fragalà, M.E. ZnTPPS demetalation: Role of polyelectrolytes on aggregation after protonation in acid. J. Porphyr. Phthalocyanines 2017, 21, 426–430.
- Romeo, A.; Castriciano, M.A.; Zagami, R.; Pollicino, G.; Monsù Scolaro, L.; Pasternack, R.F. Effect of zinc cations on the kinetics of supramolecular assembly and the chirality of porphyrin J-aggregates. Chem. Sci. 2017, 8, 961–967.
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-based nanostructures for sensing applications. J. Sens. 2009, 2009.
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583.
- Panda, M.K.; Ladomenou, K.; Coutsolelos, A.G. Porphyrins in bio-inspired transformations: Light-harvesting to solar cell. Coord. Chem. Rev. 2012, 256, 2601–2627.
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310.
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603.
- Kano, K. Porphyrin-cyclodextrin supramolecular complexes as myoglobin model in water. Colloid Polym. Sci. 2008, 286, 79–84.
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of singlet-oxygen and superoxide-ion generation by porphyrins and bacteriochlorins and their implications in photodynamic therapy. Chem. Eur. J. 2010, 16, 9273–9286.
- Mateos-Timoneda, M.A.; Crego-Calama, M.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372.
- Huang, X.; Fujioka, N.; Pescitelli, G.; Koehn, F.E.; Williamson, R.T.; Nakanishi, K.; Berova, N. Absolute configurational assignments of secondary amines by CD-sensitive dimeric zinc porphyrin host. J. Am. Chem. Soc. 2002, 124, 10320–10335.
- Saha, B.; Ikbal, S.A.; Petrovic, A.G.; Berova, N.; Rath, S.P. Complexation of Chiral Zinc-Porphyrin Tweezer with Achiral Diamines: Induction and Two-Step Inversion of Interporphyrin Helicity Monitored by ECD. Inorg. Chem. 2017, 56, 3849–3860.
- Kurtán, T.; Nesnas, N.; Li, Y.Q.; Huang, X.; Nakanishi, K.; Berova, N. Chiral recognition by CD-sensitive dimeric zinc porphyrin host. 1. Chiroptical protocol for absolute configurational assignments of monoalcohols and primary monoamines. J. Am. Chem. Soc. 2001, 123, 5962–5973.
- Proni, G.; Pescitelli, G.; Huang, X.; Quraishi, N.Q.; Nakanishi, K.; Berova, N. Configurational assignment of α-chiral carboxylic acids by complexation to dimeric Zn-porphyrin: Host-guest structure, chiral recognition and circular dichroism. Chem. Commun. 2002, 2, 1590–1591.
- Ishii, H.; Chen, Y.; Miller, R.A.; Karady, S.; Nakanishi, K.; Berova, N. Chiral recognition of cyclic α-hydroxyketones by CD-sensitive zinc tetraphenylporphyrin tweezer. Chirality 2005, 17, 305–315.
- Angelini, N.; Micali, N.; Mineo, P.; Scamporrino, E.; Villari, V.; Vitalini, D. Uncharged water-soluble Co(II)—Porphyrin: A receptor for aromatic α-amino acids. J. Phys. Chem. B 2005, 109, 18645–18651.
- Borovkov, V.V.; Lintuluoto, J.M.; Inoue, Y. Supramolecular chirogenesis in zinc porphyrins: Mechanism, role of guest structure, and application for the absolute configuration determination. J. Am. Chem. Soc. 2001, 123, 2979–2989.
- Gholami, H.; Anyika, M.; Zhang, J.; Vasileiou, C.; Borhan, B. Host–Guest Assembly of a Molecular Reporter with Chiral Cyanohydrins for Assignment of Absolute Stereochemistry. Chem. Eur. J. 2016, 22, 9235–9239.
- Borovkov, V. Supramolecular chirality in porphyrin chemistry. Symmetry 2014, 6, 256–294.
- Ferrand, Y.; Le Maux, P.; Simonneaux, G. Highly enantioselective synthesis of cyclopropylphosphonates catalyzed by chiral ruthenium porphyrins. Org. Lett. 2004, 6, 3211–3214.
- Ogoshi, H.; Mizutani, T. Multifunctional and Chiral Porphyrins: Model Receptors for Chiral Recognition. Acc. Chem. Res. 1998, 31, 81–89.
- Konishi, K.; Takahata, Y.; Aida, T.; Inoue, S.; Kuroda, R. Chiral N-Substituted Porphyrins Related to Heme Inactivation Products. First Crystallographic Determination of Absolute Stereochemistry and Correlation with Circular Dichroism. J. Am. Chem. Soc. 1993, 115, 1169–1170.
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73.
- Zhang, Z.; Hu, C.; Wang, Y.; Lang, J.-P. Crystallographic and DFT studies on host-guest complexes consisting of zinc bisporphyrinates and 1-phenylethylamine. J. Coord. Chem. 2019, 72, 1156–1170.
- Zhuo, C.-C.; Li, L.; Hu, C.-J.; Lang, J.-P. Host-guest assembly for highly sensitive probing of a chiral mono-alcohol with a zinc trisporphyrinate. Sci. Rep. 2017, 7, 3829.
- Hu, Q.; Zhuo, C.; Wang, Y.; Hu, C.; Lang, J. Chirality Transfer from Chiral Monoamines to an m-Phthalic Diamide-Linked Zinc Bisporphyrinate with a Benzylamide Substituent. Inorg. Chem. 2017, 56, 10204–10214.
- Hu, T.; Hu, C.; Wang, Y.; Young, D.J.; Lang, J.-P. Stoichiometrically controlled chirality inversion in zinc bisporphyrinate–monoamine complexes. Dalt. Trans. 2018, 47, 5503–5512.
- Hu, T.; Liu, T.; Zhang, Z.; Wang, Y.; Yang, Y.; Young, D.J.; Hu, C.; Lang, J.-P. Precise control of chirality transfer by adjusting the alkyl substituents of guests. Dye. Pigment. 2019, 160, 692–699.
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73.
- Zhou, Y.; Yu, B.; Levon, K. Potentiometric sensing of chiral amino acids. Chem. Mater. 2003, 15, 2774–2779.
- Cavazzini, A.; Nadalini, G.; Dondi, F.; Gasparrini, F.; Ciogli, A.; Villani, C. Study of mechanisms of chiral discrimination of amino acids and their derivatives on a teicoplanin-based chiral stationary phase. J. Chromatogr. A 2004, 1031, 143–158.
- Fu, Y.; Han, Q.; Chen, Q.; Wang, Y.; Zhou, J.; Zhang, Q. A new strategy for chiral recognition of amino acids. Chem. Commun. 2012, 48, 2322–2324.
- Carmo dos Santos, N.A.; Badetti, E.; Licini, G.; Abbate, S.; Longhi, G.; Zonta, C. A stereodynamic fluorescent probe for amino acids. Circular dichroism and circularly polarized luminescence analysis. Chirality 2018, 30, 65–73.
- Friedman, M.; Cuq, J.L. Chemistry, analysis, nutritional value, and toxicology of tryptophan in food. A review. J. Agric. Food Chem. 1988, 36, 1079–1093.
- Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis. Electrophoresis 2006, 27, 283–303.
- Herrero, M.; Ibáñez, E.; Martín-Álvarez, P.J.; Cifuentes, A. Analysis of chiral amino acids in conventional and transgenic maize. Anal. Chem. 2007, 79, 5071–5077.
- Kuroda, Y.; Kato, Y.; Higashioji, T.; Ogoshi, H. New Chiral Porphyrins—Syntheses and Molecular Recognition of Amino Acid Esters. Angew. Chem. Int. Ed. Engl. 1993, 32, 723–724.
- Yasuhisa Kuroda, R.; Kato, Y.; Higashioji, T.; Hasegawa, J.; Kawanami, S.; Takahashi, M.; Shiraishi, N.; Tanabe, K.; Ogoshi, H. Chiral Amino Acid Recognition by a Porphyrin-Based Artificial. J. Am. Chem. Soc 1995, 117, 10950–10958.
- Mizutani, E.T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H. Design and Synthesis of a Trifunctional Chiral Porphyrin with C2 Symmetry as a Chiral Recognition Host for Amino Acid. J. Am. Chem. Soc 1994, 116, 4240–4250.
- Mizutani, T.; Ema, T.; Yoshida, T.; Kuroda, Y.; Ogoshi, H. Recognition of α-Amino Acid Esters by Zinc Porphyrin Derivatives via Coordination and Hydrogen Bonding Interactions. Evidence for Two-Point Fixation from Thermodynamic and Induced Circular Dichroism Spectroscopic Studies. Inorg. Chem. 1993, 32, 2072–2077.
- Imai, H.; Munakata, H.; Uemori, Y.; Sakura, N. Chiral Recognition of Amino Acids and Dipeptides by a Water-Soluble Zinc Porphyrin. Inorg. Chem. 2004, 43, 1211–1213.
- Fa, H.B.; Zhao, L.; Wang, X.Q.; Yu, J.H.; Huang, Y.B.; Yang, M.; Wang, D.J. Chiral recognition of mesoporous SBA-15 with an incorporated chiral porphyrin. Eur. J. Inorg. Chem. 2006, 2006, 4355–4361.
- Crossley, M.J.; Hambley, T.W.; MacKay, L.G.; Try, A.C.; Walton, R. Porphyrin analogues of Tröger’s base: Large chiral cavities with a bimetallic binding site. J. Chem. Soc. Chem. Commun. 1995, 1077–1079.
- Crossley, M.J.; MacKay, L.G.; Try, A.C. Enantioselective recognition of histidine and lysine esters by porphyrin chiral clefts and detection of amino acid conformations in the bound state. J. Chem. Soc. Chem. Commun. 1995, 1925–1927.
- Wu, X.; Starnes, S.D. L-nipecotic acid-porphyrin derivative: A chiral host with introverted functionality for chiral recognition. Org. Lett. 2012, 14, 3652–3655.
- Ema, T.; Ouchi, N.; Doi, T.; Korenaga, T.; Sakai, T. Highly sensitive chiral shift reagent bearing two zinc porphyrins. Org. Lett. 2005, 7, 3985–3988.
- Lu, W.; Yang, H.; Li, X.; Wang, C.; Zhan, X.; Qi, D.; Bian, Y.; Jiang, J. Chiral Discrimination of Diamines by a Binaphthalene-Bridged Porphyrin Dimer. Inorg. Chem. 2017, 56, 8223–8231.
- Easson, L.H.; Stedman, E. Studies on the relationship between chemical constitution and physiological action. Biochem. J. 1933, 27, 1257–1266.
- Ogston, A.G. Interpretation of experiments on metabolic processes, using isotopic tracer elements [9]. Nature 1949, 163, 963.
- Berthod, A. Chiral recognition mechanisms. Anal. Chem. 2006, 78, 2093–2099.
