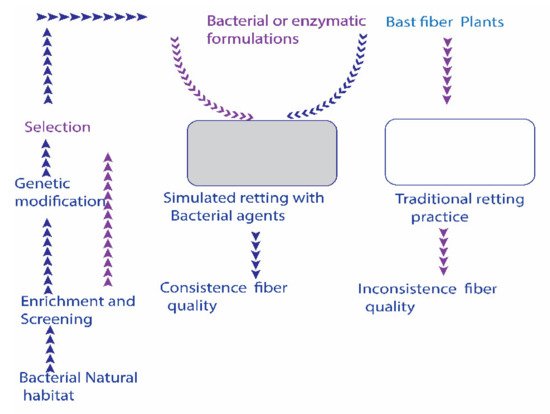
Bast fiber plants require a post-harvest process to yield useable natural cellulosic fibers, denoted as retting or degumming. It encompasses the degradation of the cell wall’s non-cellulosic gummy substances (NCGs), facilitating fibers separations, setting the fiber’s quality, and determining downstream usages. Due to the inconvenience of traditional retting practices, bacterial inoculum and enzyme applications for retting gained attention. Therefore, concurrent changes of agroclimatic and socioeconomic conditions, the conventional water retting confront multiple difficulties, bast industries become vulnerable, and bacterial agents mediated augmented bio-retting processes trying to adapt to sustainability. However, this process’s success demands a delicate balance among substrates and retting-related biotic and abiotic factors. These critical factors were coupled to degrade bast fibers NCGs in bacterial retting while holistically disregarded in basic research. In this study, a set of factors were defined that critically regulates the process and requires to be comprehended to achieve optimum retting without failure.
- non-cellulosic gums
- bacterial retting
- degumming enzymes
- pectin esterification
- retting factors
- macerating solvent
1. Introduction
2. Bacterial Retting Process
3. Bast Fiber Cell Wall Structure Influencing Bacterial Retting
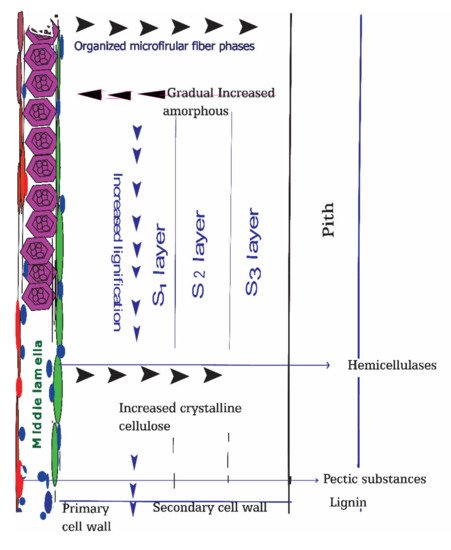
4. Bast Fibers Cell Walls Constituents Affecting Retting Process
5. Bacterial Strains Affecting Retting Process
| Bacteria | Strain | Enzymes | Optimum pH | Optimum Temp. | Ref. |
|---|---|---|---|---|---|
| Bacillus sp. | NT-39, 53, 76 MG-cp-2 |
PL, XL, PG, Lac |
10 | 45 °C, 60 °C | [51][52][53] |
| B. Pumilus | NRRL B-212, ATCC 7061 |
Exo-pectinase | - | - | [54][55] |
| B. subtilis | EFRL 01 | PG | 8 | 45 °C | [56] |
| B. licheniformis | HDYM-03 KIBGE IB-21, SHG10, DSM-13 |
PL, PG, PNL | - | - | [57][58][59][60] |
| B. Cereus | HDYM-02 | PG | - | - | [61][62][63] |
| B. megaterium | HDYM-09 AK2 |
PNL | - | - | [57][64] |
| B. clausii | - | - | - | - | [65] |
| B. tequilensis | SV11-UV37 CAS-MEI-2-33 |
PL, PG P |
10 | 40 °C | [66][67] |
| B. halodurans | M29 | - | - | [68] | |
| B. mojavensis | M14, | - | - | - | [69] |
| Enterobacteriaceae Pectobacterium sp., Enterobacter lignolyticus | DCE-01, SCF1 | P, MN, XL Lac |
- | 35 °C | [70][71] |
| Bacterial Consortia | Composition | Enzymatic Potentials | Ref. |
|---|---|---|---|
| CRIJAF SONA | Bacillus sp (PJRB1—Acc. No. MTCC 5573, PJRB2-MTCC 5574, and PJRB3-MTCC 5575) | PG, PNL and Xylanase | [73] |
| RAMCD407 | P. aeruginosa, Enterococcus sp., B. subtilis, Bacillus sp. | Pectinase, xylanase and mananase | [22] |
| C-51, C-67, and C-90 | B. megaterium, B. subtilis, B.s cereus, B. xiamenensis, B. koreensis, P. mirabilis, E. tabaci, K. oryzae, S. nematodiphila and Aeromonasjandaei. | Pectinase and Xylanase | [72] |
| MC1, MC2, MC3 | B. subtilis B.s pumilus IMAU80221, B. pumilus GVC11 and B. pumilus SYBC-W | Pectinase | [16][20] |
6. Bacterial Retting Enzymes Affecting Retting Process
| Name of Enzymes | Enzyme Types | Common Name | E. C. Suggested No. |
Substrate | Cleavage Mechanism | Product |
|---|---|---|---|---|---|---|
| Pectinases (Depolymerizes) | Hydrolases | Exo- polygalacturonases (Exo-PG1 or PG |
3.2.1.67 | Pectate | Catalyze the α-1,4 glycosidic linkage | mono galacturonate |
| Exo- polygalacturonases (Exo-PG2 or P.G.) |
3.2.1.82 | Pectate | Act on end cleaving site | Di galacturonate’s | ||
| Lyases: (Polygalacturonate Lyase (PGL) | Pectate lyases (Exo-PGL or P.L.) | 4.2.2.9 | Pectin | Act on end site bonds | Unsaturated Di galacturonate’s | |
| Lyases: Polymethyl galacturonate Lyase (PMGL) |
Pectin lyases (Exo-PMGL or PNL) |
4.2.2.10 | Pectin | Random cleavage | Unsaturated methyl oligo galacturonate | |
| Pectinases (De-esterifying) |
Esterase | Polymethyl galacturonate esterase (PMGE or PME) | 3.1.1.11 | Pectin | Random modification of ester group | Pectin acid and methanol |
| Hemicellulose | Hydrolases | Xylanase | 3.1.1.8 | Xylan Mannan |
Cleavage β-1,4 glycosidic linkage | xylose |
| Cellulase | Hydrolases | Endoglucanase | 3.2.1.4 | Amorphous Cellulose |
Nonreducing end of the cellulose chain | glucose |
| Laccase | Peroxidases | Laccase Mn peroxidase |
1.10.3.2 1.11.1.13 |
Lignin | - | p-quinone |
| Product | Producers | Activities |
|---|---|---|
| ScourzymeL | Novozymes | PG, PL |
| Bioprep 3000L | Novozymes | P |
| Texazym BFE | Inotex | P |
| Texazym SCW INOTEX Ltd., | Inotex | P, Xy, EG |
| Texazym DLG | Inotex. | Xy, CeB, EG, LPO |
| Texazym SER-3 | Inotex | P, Xy, EG |
| Texazym SER-4 | inotex | P, Xy, EG, CeB |
| Texazym SER-5 | inotex | P, Xy, EG, CeB and Lip |
| Baylase EVO | Lanxess | P |
| Lyvelin | Lyven | P |
7. Interaction of Others Retting Factors
This entry is adapted from the peer-reviewed paper 10.3390/fib9080052
References
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre-based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584.
- Zimniewska, M.; Wladyka-Przybylak, M.; Mankowski, J. Cellulosic bast fibers, their structure and properties suitable for composite applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; pp. 97–119.
- Shekar, H.S.; Ramachandra, M. Green composites: A review. Mater. Today Proc. 2018, 5, 2518–2526.
- Sisti, L.; Totaro, G.; Vannini, M.; Celli, A. Retting process as a pretreatment of natural fibers for the development of polymer composites. In Smart Polymer Nanocomposites; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 97–135.
- Tezara, C.; Siregar, J.P.; Lim, H.; Fauzi, F.; Yazdi, M.H.; Moey, L.; Lim, J. Factors that affect the mechanical properties of kenaf fiber reinforced polymer: A review. J. Mech. Eng. Sci. 2016, 10, 2159–2175.
- Sisti, L.; Totaro, G.; Vannini, M.; Fabbri, P.; Kalia, S.; Zatta, A.; Celli, A. Evaluation of the retting process as a pre-treatment of vegetable fibers for the preparation of high-performance polymer biocomposites. Ind. Crop. Prod. 2016, 81, 56–65.
- Han, G.; Jiang, W.; Li, X.; Zhang, X.; Zhang, Y.; Li, M. Effect of steam pressure on chemical and structural properties of kenaf fibers during steam explosion process. BioResources 2016, 11, 6590–6599.
- Keller, A.; Leupin, M.; Mediavilla, V.; Wintermantel, E. Influence of the growth stage of industrial hemp on chemical and physical properties of the fibres. Ind. Crop. Prod. 2001, 13, 35–48.
- Mediavilla, V.; Leupin, M.; Keller, A. Influence of the growth stage of industrial hemp on the yield formation in relation to certain fibre quality traits. Ind. Crop. Prod. 2001, 13, 49–56.
- Mazzanti, V.; Pariante, R.; Bonanno, A.; de Ballesteros, O.R.; Mollica, F.; Filippone, G. Reinforcing mechanisms of natural fibers in green composites: Role of fibers morphology in a PLA/hemp model system. Compos. Sci. Technol. 2019, 180, 51–59.
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gamez-Perez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225.
- Santoni, A.; Bonfiglio, P.; Fausti, P.; Marescotti, C.; Mazzanti, V.; Mollica, F.; Pompoli, F. Improving the sound absorption performance of sustainable thermal insulation materials: Natural hemp fibres. Appl. Acoust. 2019, 150, 279–289.
- Fernando, D.; Thygesen, A.; Meyer, A.S.; Daniel, G. Elucidating field retting mechanisms of hemp fibres for biocomposites: Effects of microbial actions and interactions on the cellular micro-morphology and ultrastructure of hemp stems and bast fibres. BioResources 2019, 14, 4047–4084.
- Ahmed, Z.; Nizam, S.A. Jute—Microbiological and biochemical research. Plant Tissue Cult. Biotechnol. 2008, 18, 197–220.
- Majumdar, B.; Chattopadhyay, L.; Barai, S.; Saha, A.R.; Sarkar, S.; Mazumdar, S.P.; Saha, R.; Jha, S.K. Impact of conventional retting of jute (Corchorus spp.) on the environmental quality of water: A case study. Environ. Monit. Assess. 2019, 191, 440.
- Das, B.; Chakrabarti, K.; Ghosh, S.; Majumdar, B.; Tripathi, S.; Chakraborty, A. Effect of efficient pectinolytic bacterial isolates on retting and fibre quality of jute. Ind. Crop. Prod. 2012, 36, 415–419.
- Paridah, M.T.; Basher, A.B.; SaifulAzry, S.; Ahmed, Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources 2011, 6, 5260–5281.
- Liu, M.; Ale, M.T.; Kołaczkowski, B.; Fernando, D.; Daniel, G.; Meyer, A.S.; Thygesen, A. Comparison of traditional field retting and Phlebia radiata Cel 26 retting of hemp fibres for fibre-reinforced composites. AMB Express 2017, 7, 1–15.
- Kavuthodi, B.; Sebastian, D. Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: Media formulation and statistical optimization for submerged fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 715–722.
- Das, S.; Majumdar, B.; Saha, A.R. Biodegradation of Plant Pectin and Hemicelluloses with Three Novel Bacillus pumilus Strains and Their Combined Application for Quality Jute Fibre Production. Agric. Res. 2015, 4, 354–364.
- Chauhan, S.; Sharma, A.K.; Jain, R.K. Enzymatic retting: A revolution in the handmade papermaking from Calotropis procera. In Biotechnology for Environmental Management and Resource Recovery; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; pp. 77–88.
- Mao, K.; Chen, H.; Qi, H.; Qiu, Z.; Zhang, L.; Zhou, J. Visual degumming process of ramie fiber using a microbial consortium RAMCD407. Cellulose 2019, 26, 3513–3528.
- Tamburini, E.; Leon, A.G.; Perito, B.; Mastromei, G. Characterization of bacterial pectinolytic strains involved in the water retting process. Environ. Microbiol. 2003, 5, 730–736.
- Jankauskienė, Z.; Butkutė, B.; Gruzdevienė, E.; Ceseviciene, J.; Fernando, A.L. Chemical composition and physical properties of dew- and water-retted hemp fibers. Ind. Crop. Prod. 2015, 75, 206–211.
- Gorshkova, T.A.; Gurjanov, O.P.; Mikshina, P.; Ibragimova, N.N.; Mokshina, N.E.; Salnikov, V.V.; Ageeva, M.V.; Amenitskii, S.I.; Chernova, T.E.; Chemikosova, S.B. Specific type of secondary cell wall formed by plant fibers. Russ. J. Plant Physiol. 2010, 57, 328–341.
- Hobson, N.; Roach, M.J.; Deyholos, M.K. Gene expression in tension wood and bast fibres. Russ. J. Plant Physiol. 2010, 57, 321–327.
- Gurjanov, O.P.; Ibragimova, N.N.; Gnezdilov, O.; Gorshkova, T. Polysaccharides, tightly bound to cellulose in cell wall of flax bast fibre: Isolation and identification. Carbohydr. Polym. 2008, 72, 719–729.
- Liu, M.; Fernando, D.; Daniel, G.; Madsen, B.; Meyer, A.S.; Ale, M.T.; Thygesen, A. Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind. Crop. Prod. 2015, 69, 29–39.
- Abbott, D.W.; Boraston, A.B. Structural biology of pectin degradation by enterobacteriaceae. Microbiol. Mol. Biol. Rev. 2008, 72, 301–316.
- Prasad, B.M.; Sain, M.M. Mechanical properties of thermally treated hemp fibers in inert atmosphere for potential composite reinforcement. Mater. Res. Innov. 2003, 7, 231–238.
- Gorshkova, T.; Brutch, N.; Chabbert, B.; Deyholos, M.; Hayashi, T.; Lev-Yadun, S.; Mellerowicz, E.J.; Morvan, C.; Neutelings, G.; Pilate, G. Plant fiber formation: State of the art, recent and expected progress, and open questions. Crit. Rev. Plant Sci. 2012, 31, 201–228.
- Mokshina, N.; Chernova, T.; Galinousky, D.; Gorshkov, O.; Gorshkova, T. Key stages of fiber development as determinants of bast fiber yield and quality. Fibers 2018, 6, 20.
- Meshram, J.H.; Palit, P. Biology of industrial bast fibers with reference to quality. J. Nat. Fibers 2013, 10, 176–196.
- Ouajai, S.; Shanks, R. Solvent and enzyme induced recrystallization of mechanically degraded hemp cellulose. Cellulose 2006, 13, 31–44.
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779.
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576.
- Henriksson, G.; Akin, D.E.; Slomczynski, D.; Eriksson, K.-E.L. Production of highly efficient enzymes for flax retting by Rhizomucor pusillus. J. Biotechnol. 1999, 68, 115–123.
- Komuraiah, A.; Kumar, N.S.; Prasad, B.D. Chemical composition of natural fibers and its influence on their mechanical properties. Mech. Compos. Mater. 2014, 50, 359–376.
- Mellerowicz, E.J.; Sundberg, B. Wood cell walls: Biosynthesis, developmental dynamics and their implications for wood properties. Curr. Opin. Plant Biol. 2008, 11, 293–300.
- Vignon, M.; Dupeyre, D.; Garcia-Jaldon, C. Morphological characterization of steam-exploded hemp fibers and their utilization in polypropylene-based composites. Bioresour. Technol. 1996, 58, 203–215.
- Vignon, M.R.; Garcia-Jaldon, C. Structural features of the pectic polysaccharides isolated from retted hemp bast fibres. Carbohydr. Res. 1996, 296, 249–260.
- Yu, H.; Yu, C. Influence of various retting methods on properties of kenaf fiber. J. Text. Inst. 2010, 101, 452–456.
- Schols, H.; Coenen, G.; Voragen, A. Revealing Pectin’s Structure; Pectins and Pectinases: Wageningen, The Netherlands, 2009; pp. 19–34.
- Aritkhodzhaev, K.A.; Arifkhodzhaev, A.O.; Yusupov, A.M. Pectin from green kenaf bast. Chem. Nat. Compd. 1995, 31, 159–162.
- Day, A.; Ruel, K.; Neutelings, G.; Crônier, D.; David, H.; Hawkins, S.; Chabbert, B. Lignification in the flax stem: Evidence for an unusual lignin in bast fibers. Planta 2005, 222, 234–245.
- Suzuki, H.; Macdonald, J.; Syed, K.; Salamov, A.; Hori, C.; Aerts, A.; Henrissat, B.; Wiebenga, A.; Vankuyk, P.A.; Barry, K.; et al. Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genom. 2012, 13, 444.
- Ajith, S. Bacterial degradation of lignin: A prospective for lignocellulosic biofuels. Int. J. Innov. Sci. Res. Technol. 2019, 4, 11.
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Compos. 2008, 29, 187–207.
- MacDonald, J.; Doering, M.; Canam, T.; Gong, Y.; Guttman, D.S.; Campbell, M.M.; Master, E.R. Transcriptomic responses of the softwood-degrading white-rot fungus Phanerochaete carnosa during growth on coniferous and deciduous wood. Appl. Environ. Microbiol. 2011, 77, 3211–3218.
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635.
- Zhang, J.; Henriksson, G.; Johansson, G. Polygalacturonase is the key component in enzymatic retting of flax. J. Biotechnol. 2000, 81, 85–89.
- Azeri, C.; Tamer, U.; Oskay, M. Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr. J. Biotechnol. 2010, 9, 1684–5315.
- Kapoor, M.; Beg, Q.K.; Bhushan, B.; Singh, K.; Dadhich, K.; Hoondal, G. Application of an alkaline and thermostable polygalacturonase from Bacillus sp. MG-cp-2 in degumming of ramie (Boehmeria nivea) and sunn hemp (Crotalaria juncea) bast fibres. Process. Biochem. 2001, 36, 803–807.
- Tepe, O.; Dursun, A.Y. Exo-pectinase production by Bacillus pumilus using different agricultural wastes and optimizing of medium components using response surface methodology. Environ. Sci. Pollut. Res. 2014, 21, 9911–9920.
- Liang, C.; Gui, X.; Zhou, C.; Xue, Y.; Ma, Y.; Tang, S.-Y. Improving the thermoactivity and thermostability of pectate lyase from Bacillus pumilus for ramie degumming. Appl. Microbiol. Biotechnol. 2014, 99, 2673–2682.
- Qureshi, A.S.; Bhutto, M.A.; Chisti, Y.; Khushk, I.; Dahot, M.U.; Bano, S. Production of pectinase by Bacillus subtilis EFRL 01 in a date syrup medium. Afr. J. Biotechnol. 2012, 11, 12563–12570.
- Ge, J.; Yang, Z.; Du, R.; Zhang, L.; Ping, W.; Zhao, D. Production of pectinolytic enzymes by two Bacillus spp. strains and their application in flax degumming. Trans. Tianjin Univ. 2019, 25, 413–419.
- Rehman, H.U.; Aman, A.; Nawaz, M.A.; Qader, S.A.U. Characterization of pectin degrading polygalacturonase produced by Bacillus licheniformis KIBGE-IB21. Food Hydrocoll. 2015, 43, 819–824.
- Embaby, A.M.; Masoud, A.A.; Marey, H.S.; Shaban, N.Z.; Ghonaim, T.M. Raw agro-industrial orange peel waste as a low cost effective inducer for alkaline polygalacturonase production from Bacillus licheniformis SHG10. SpringerPlus 2014, 3, 327.
- Remoroza, C.; Wagenknecht, M.; Buchholt, H.C.; Moerschbacher, B.M.; Gruppen, H.; Schols, H.A. Mode of action of Bacillus licheniformis pectin methylesterase on highly methylesterified and acetylated pectins. Carbohydr. Polym. 2015, 115, 540–550.
- Zhao, D.; Liu, P.; Pan, C.; Du, R.; Ping, W.; Ge, J. Bacterial succession and metabolite changes during flax (Linum usitatissimum L.) retting with Bacillus cereus HDYM-02. Sci. Rep. 2016, 6, 31812.
- Torimiro, N.; Okonji, R. A comparative study of pectinolytic enzyme production by Bacillus species. Afr. J. Biotechnol. 2013, 12, 6498–6503.
- Namasivayam, E.; Ravindar, J.; Mariappan, K.; Akhil, J.; Mukesh, K.; Jayaraj, R. Production of extracellular pectinase by Bacillus cereus isolated from market solid waste. J. Bioanal. Biomed. 2011, 3, 70–75.
- Mukhopadhyay, A.; Dutta, N.; Chattopadhyay, D.; Chakrabarti, K. Degumming of ramie fiber and the production of reducing sugars from waste peels using nanoparticle supplemented pectate lyase. Bioresour. Technol. 2013, 137, 202–208.
- Liu, Y.; Chen, G.; Wang, J.; Hao, Y.; Li, M.; Li, Y.; Hu, B.; Lu, F. Efficient expression of an alkaline pectate lyase gene from Bacillus subtilis and the characterization of the recombinant protein. Biotechnol. Lett. 2011, 34, 109–115.
- Zhang, G.; Li, S.; Xu, Y.; Wang, J.; Wang, F.; Xin, Y.; Shen, Z.; Zhang, H.; Ma, M.; Liu, H. Production of alkaline pectinase: A case study investigating the use of tobacco stalk with the newly isolated strain Bacillus tequilensis CAS-MEI-2-33. BMC Biotechnol. 2019, 19, 45.
- Chiliveri, S.R.; Linga, V.R. A novel thermostable, alkaline pectate lyase from Bacillus tequilensis SV11 with potential in textile industry. Carbohydr. Polym. 2014, 111, 264–272.
- Mei, Y.; Chen, Y.; Zhai, R.; Liu, Y. Cloning, purification and biochemical properties of a thermostable pectinase from Bacillus halodurans M29. J. Mol. Catal. B Enzym. 2013, 94, 77–81.
- Ghazala, I.; Sayari, N.; Ben Romdhane, M.; Ellouz-Chaabouni, S.; Haddar, A. Assessment of pectinase production by Bacillus mojavensis I4 using an economical substrate and its potential application in oil sesame extraction. J. Food Sci. Technol. 2015, 52, 7710–7722.
- Duan, S.; Cheng, L.; Liu, Z.; Feng, X.; Zheng, K.; Peng, Y. Diversity and characteristics of kenaf bast degumming microbial resources. J. Nat. Fibers 2018, 15, 799–807.
- DeAngelis, K.M.; D’Haeseleer, P.; Chivian, D.; Fortney, J.L.; Khudyakov, J.; Simmons, B.; Woo, H.; Arkin, A.P.; Davenport, K.W.; Goodwin, L. Complete genome sequence of “Enterobacter lignolyticus” SCF1. Stand. Genom. Sci. 2011, 5, 69–85.
- Hasan, R.; Aktar, N.; Kabir, S.M.T.; Honi, U.; Halim, A.; Islam, R.; Sarker, M.D.H.; Haque, S.; Alam, M.; Islam, S. Pectinolytic bacterial consortia reduce jute retting period and improve fibre quality. Sci. Rep. 2020, 10, 5174.
- Datta, S.; Saha, D.; Chattopadhyay, L.; Majumdar, B. Genome comparison identifies different Bacillus species in a bast fibre-retting bacterial consortium and provides insights into pectin degrading genes. Sci. Rep. 2020, 10, 8169.
- Bugg, T.D.H.; Rahmanpour, R.; Rashid, G.M.M. Bacterial enzymes for lignin oxidation and conversion to renewable chemicals. In Production of Biofuels and Chemicals from Lignin; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 131–146.
- Wu, Y.-R.; He, J. Characterization of anaerobic consortia coupled lignin depolymerization with biomethane generation. Bioresour. Technol. 2013, 139, 5–12.
- Chen, Y.; Chai, L.; Zhu, Y.; Yang, Z.; Zheng, Y.; Zhang, H. Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J. Appl. Microbiol. 2012, 112, 900–906.
- Lee, C.H.; Khalina, A.; Lee, S.H.; Liu, M. A Comprehensive review on bast fibre retting process for optimal performance in fibre-reinforced polymer composites. Adv. Mater. Sci. Eng. 2020, 2020, 6074063.
- Antonov, V.; Marek, J.; Bjelkova, M.; Smirous, P.; Fischer, H. Easily available enzymes as natural retting agents. Biotechnol. J. 2007, 2, 342–346.
- Dreyer, J.; Müssig, J.; Koschke, N.; Ibenthal, W.-D.; Harig, H. Comparison of enzymatically separated hemp and nettle fibre to chemically separated and steam exploded hemp fibre. J. Ind. Hemp 2002, 7, 43–59.
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van De Voorde, I. Cost-efficient enzymatic extraction, and treatment of natural fibres for high quality composites. In Proceedings of the MultiHemp Fibre Quality Workshop, Villeneuve d’Ascq, France, 18 January 2018.
- Abdullah, S.; Zuhudi, N.; Anuar, N.; Isa, M. Mechanical and thermal characterization of alkali treated kenaf fibers. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Batu Ferringhi, Penang, Malaysia, 21–22 November 2017; p. 012048.
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596.
- Réquilé, S.; Le Duigou, A.; Bourmaud, A.; Baley, C. Peeling experiments for hemp retting characterization targeting biocomposites. Ind. Crop. Prod. 2018, 123, 573–580.
- Favela-Torres, E.; Volke-Sepúlveda, T.; Viniegra-González, G. Production of hydrolytic depolymerising pectinases. Food Technol. Biotechnol. 2006, 44, 221–227.
- Ovodov, Y.S. Current views on pectin substances. Russ. J. Bioorganic Chem. 2009, 35, 269–284.
- Song, K.H.; Obendorf, S.K. Chemical and biological retting of kenaf fibers. Text. Res. J. 2006, 76, 751–756.
- Nacos, M.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.; Christakopoulos, P.; Polissiou, M. Kenaf xylan—A source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134.
- Kashyap, D.; Vohra, P.; Chopra, S.; Tewari, R. Applications of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227.
- Yu, P.; Zhang, Y.; Gu, D. Production optimization of a heat-tolerant alkaline pectinase from Bacillus subtilis ZGL14 and its purification and characterization. Bioengineered 2017, 8, 613–623.
- Li, X.; Wang, H.; Zhou, C.; Ma, Y.; Li, J.; Song, J. Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnol. 2014, 14, 18.
- Saha, M.; Rana, R.S.; Adhikary, B.; Mitra, S. Screening of bacterial strains for pectate lyase production and detection of optimal growth conditions for enhanced enzyme activity. J. Appl. Nat. Sci. 2017, 9, 370–374.
- Boisset, C.; Chanzy, H.; Henrissat, B. Optimized mixtures of recombinantHumicola insolens cellulases for the biodegradation of crystalline cellulose. Biotechnol. Bioeng. 2000, 72, 339–345.
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Matsushima, C.; Doi, R.H.; Ueda, M. Comparison of the mesophilic cellulosome-producing Clostridium cellulovorans genome with other cellulosome-related clostridial genomes. Microb. Biotechnol. 2010, 4, 64–73.
- Xu, R.; Zhang, K.; Liu, P.; Han, H.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X. Lignin depolymerization and utilization by bacteria. Bioresour. Technol. 2018, 269, 557–566.
- Huang, X.-F.; Santhanam, N.; Badri, D.V.; Hunter, W.J.; Manter, D.K.; Decker, S.R.; Vivanco, J.M.; Reardon, K.F. Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol. Bioeng. 2013, 110, 1616–1626.
- Razali, N.; Salit, M.S.; Jawaid, M.; Ishak, M.R.; Lazim, Y. A study on chemical composition, physical, tensile, morphological, and thermal properties of roselle fibre: Effect of fibre maturity. BioResources 2014, 10, 1803–1824.
- Das, B.; Chakrabarti, K.; Ghosh, S.; Chakraborty, A.; Saha, M.N. Assessment of changes in community level physiological profile and molecular diversity of bacterial communities in different stages of jute retting. Pak. J. Biol. Sci. 2013, 16, 1722–1729.
- Liu, X.; Kokare, C. Microbial enzymes of use in industry. In Biotechnology of Microbial Enzymes; Academic Press: Cambridge, MA, USA, 2017; pp. 267–298.
- Djemiel, C.; Grec, S.; Hawkins, S. Characterization of bacterial and fungal community dynamics by high-throughput sequencing (HTS) metabarcoding during flax dew-retting. Front. Microbiol. 2017, 8, 2052.
- Majumdar, B.; Das, S.; Saha, A.; Chowdhury, H.; Maitra, D.; Saha, M. Improved Retting of Jute and Mesta with Microbial Formulation; ICAR-Central Research Institute for Jute and Allied Fibres: Kolkata, India, 2013.
- Kundu, A.; Chakraborty, A.; Alam Mandal, N.; Das, D.; Karmakar, P.G.; Singh, N.K.; Sarkar, D. A restriction-site-associated DNA (RAD) linkage map, comparative genomics and identification of QTL for histological fibre content coincident with those for retted bast fibre yield and its major components in jute (Corchorus olitorius L., Malvaceae s. l.). Mol. Breed. 2015, 35, 19.
- Waldron, D.; Harwood, J. A preliminary investigation into the influence of chemical composition on the dynamic mechanical properties of flax (Linum usitattisimum) straw. J. Nat. Fibers 2011, 8, 126–142.
- Lam, T.B.T.; Hori, K.; Iiyama, K. Structural characteristics of cell walls of kenaf (Hibiscus cannabinus L.) and fixation of carbon dioxide. J. Wood Sci. 2003, 49, 255–261.
- Niture, S. Comparative biochemical and structural characterizations of fungal polygalacturonases. Biologia 2008, 63, 1–19.
- Sadrmanesh, V.; Chen, Y. Bast fibres: Structure, processing, properties, and applications. Int. Mater. Rev. 2019, 64, 381–406.
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171.
- Preiss, L.; Hicks, D.B.; Suzuki, S.; Meier, T.; Krulwich, T.A. Alkaliphilic bacteria with impact on industrial applications, concepts of early life forms, and bioenergetics of ATP synthesis. Front. Bioeng. Biotechnol. 2015, 3, 75.
- Clark, J. Select the right fabric for liquid-solid separation. Chem. Eng. Prog. 1990, 86, 45–50.
- Adamsen, A.P.S.; Akin, D.E.; Rigsby, L.L. Chemical retting of flax straw under alkaline conditions. Text. Res. J. 2002, 72, 789–794.
- Krall, S.M.; McFeeters, R.F. Pectin hydrolysis: Effect of temperature, degree of methylation, pH, and calcium on hydrolysis rates. J. Agric. Food Chem. 1998, 46, 1311–1315.
- Foulk, J.; Akin, D.; Dodd, R. Influence of pectinolytic enzymes on retting effectiveness and resultant fiber properties. BioResources 2008, 3, 155–169.
- Hoondal, G.; Tiwari, R.; Tewari, R.; Dahiya, N.; Beg, Q. Microbial alkaline pectinases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2002, 59, 409–418.
- Cao, J.; Zheng, L.; Chen, S. Screening of pectinase producer from alkalophilic bacteria and study on its potential application in degumming of ramie. Enzym. Microb. Technol. 1992, 14, 1013–1016.
- Zhang, L.; Zhu, R.; Chen, J.; Feng, X. Seawater-retting treatment of hemp and characterization of bacterial strains involved in the retting process. Process. Biochem. 2008, 43, 1195–1201.
