Thyroid cancer (TC) is rare relative to cancers of many other organs (breast, prostate, lung, and colon). The majority of TCs are differentiated tumors that are relatively easy to treat and have a good prognosis. However, for anaplastic TC, a rapidly growing and aggressive tumor, treatment is suboptimal because the effective drugs cause severe adverse effects. Drug delivery by nanocarriers can improve treatment by reducing side effects. This can either be mediated through better retention in the tumor tissue due to size (passive targeting) or through the attachment of specific molecules that zero in on the cancer cells (active targeting). Nanoparticles are already used for diagnosis and imaging of TC. For unresectable anaplastic TC, nanoparticle-based treatments, less suitable for deeply located cancers, could be useful, based on low-intensity focused ultrasound and near-infrared irradiation. All potential applications of nanoparticles in TC are still in the preclinical phase.
- cancer treatment
- differentiated thyroid cancer
- anaplastic thyroid cancer
- endocrine cancer
- mesoporous silica nanoparticles
- gold nanoparticles
- thyroid imaging
- nanomedicine
- targeted therapy
1. Introduction
The World Health Organization (WHO) estimated in 2014 that the incidence of cancer will increase to 22 million annually within the next two decades and cancer deaths will rise from an estimated 8.2 million in 2012 to 13 million in 2032. These estimations are in line with data of the Global Cancer Statistics 2020 for 36 Cancers in 185 Countries that reported 19.3 million new cases and almost 10 million cancer death in 2020. Each of the common cancers (female breast, lung, and colorectal) represented 10–12% of the new cases in 2020. In contrast, the incidence of cancers of the endocrine system is relatively low, with thyroid cancer (TC) as the most common endocrine cancer, accounting for 3% of new cancer cases. The European Study RARECARE reported that the incidence of carcinomas of endocrine glands ranged from 4/100,000 person-years for TC to 2/10,000,000 person-years for parathyroid carcinoma.
2. Thyroid Carcinoma
The majority of TC are papillary carcinomas (PTC, 80%), follicular carcinomas (FTC, 10%), and medullary thyroid carcinomas (MTC, 5–10%). Anaplastic carcinomas (ATC) represent 1–2% of thyroid malignancies and primary thyroid lymphomas and primary thyroid sarcomas are extremely rare. Hürthle cell carcinoma is considered a variant of follicular carcinoma. Classification of the different subtypes of TC is outside the scope of this review and the reader is referred to expert reviews on this topic. The origins of the tumors are either the epithelial cells of the thyroid follicles (in PTC, FTC, and ATC) or the parafollicular cells, which produce the hormone calcitonin (in MTC). MTC belongs to the group of neuroendocrine tumors (NETs) derived from cells that have the unique ability to synthesize, store, and secrete a variety of metabolically active substances, peptides, and amines and can be located in various parts of the human body. The majority of the differentiated TC, PTC, and FTC are not highly aggressive and invasive tumors, and nearly all NETs of the pancreas (insulinoma, glucagonoma, and gastrinoma) and pheochromocytoma are not malignant.
The prognosis of differentiated TC is very good and in patients younger than 50 years of age, both PTC and FTC have a more than 98% cure rate. MTC have a worse prognosis because it metastasizes to lymph nodes at an early stage and requires extensive surgery. ATC is usually diagnosed after it has already spread and is one of the most incurable cancers. The tumors can be very complex, with 20–30% of ATC patients having tumor areas with ATC and PTC histology. Progression from poorly differentiated PTC to ATC has been reported in 20% of ATC patients and, according to the American Thyroid Association, is a risk factor for ATC.
Treatment of differentiated TC according to the guidelines of the European Society of Medical Oncology (ESMO) includes thyroidectomy with different extents of lymph node resection (ranging from no to radical neck dissection) followed by radioiodine treatment with 131Iodine. Radioiodine treatment can also be used as the only treatment option. Prior to the treatment, iodine uptake is stimulated either by withdrawal of levothyroxine or by stimulation with recombinant thyroid stimulating hormone (TSH). Treatment of TC has been unified based on collaboration between the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association.
Monitoring after radioiodine treatment includes thyroglobulin (Tg) and anti-Tg antibody levels, in addition to TSH levels. Tg measurement is the most sensitive parameter for persistent and/or recurrent disease. Tg antibodies can interfere with the measurement and yield evidence of functional thyroid cells. Elevated levels may indicate recurrent/metastatic disease. Neck ultrasound should also be included in routine monitoring. Whole-body scans (WBC) with a radioactive iodine tracer as well as [18F]fluorodeoxyglucose positron emission tomography (PET)/computed tomography (CT) can also be used. In the case of a suboptimal response, administration of lenvatinib and sorafenib is recommended (Figure 1).
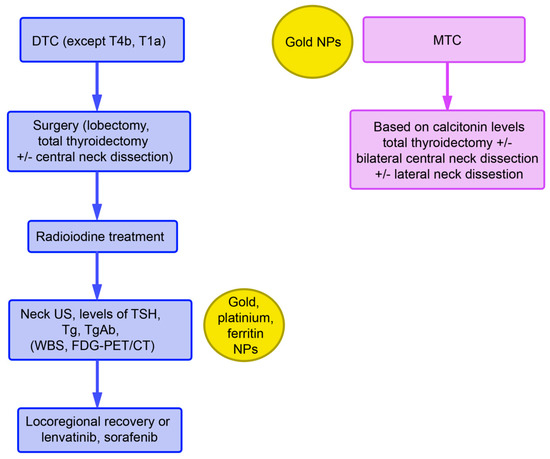
Both of these drugs are tyrosine kinase inhibitors (TKIs) for multiple receptors, which include receptor tyrosine kinases (RTK) and growth factor receptors (GFR). They inhibit kinases of the vascular endothelial growth factor receptor (VEGFR)1-3 and of RAF, V-raf murine sarcoma viral oncogene homolog B (BRAF), platelet-derived growth factor receptor (PDGFR), cKIT, FMS-like tyrosine kinase-3 (FLT3), fibroblast growth factor receptor (FGFR)1–4, and RET for proliferation and normal cell function. GFR signaling acts via the rat sarcoma virus (RAS)/RAF/Mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade (Figure 2). Binding of a ligand to RTK is the key activation step for the RAS cascade. In parallel, the ERK cascade is also activated. In turn, increased activity of RTK, RAS, and RAF activate the mitogen-activated protein kinase (MAPK) pathway, resulting in constitutive activation of MEK and ERK. Regarding the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, AKT is activated by the conversion of phosphatidylinositol bisphosphate to phosphatidylinositol trisphosphate via PI3K. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) acts as a negative regulator of this pathway by catalyzing dephosphorylation. Several TKIs inhibit the downstream steps. Dabrafenib and sorafenib for mutated BRAF and tramentinib for MEK are of relevance for ATC treatment.
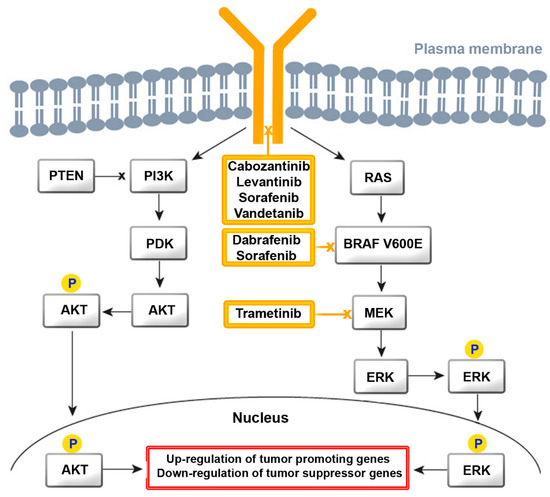
TKIs have been approved for treating differentiated TC, MTC, and ATC. Their use should be considered after careful weighing of the potential risks and benefits of this therapy. Lenvatinib has serious adverse effects on the cardiovascular system, liver, and renal function. Sorafenib use is associated with severe skin adverse events, hemorrhage, and cardiovascular effects. A systematic review by Fleeman et al. found that improvements in progression-free survival and objective tumor response rate were accompanied by increased risk of adverse effects. The effect on overall survival and health-related quality of life remains uncertain. MTCs are treated according to calcitonin levels with total thyroidectomy, bilateral central neck dissection, and lateral neck dissection. The multi-kinase TKIs vandetanib and cabozantinib show real-world efficacy and safety in the treatment of progressive MTC and will potentially be included into the recommendations of ESMO in the future.
For poorly differentiated tumors and ATC, total thyroidectomy + neck dissection followed by external beam radiotherapy (EBRT) ± chemotherapy is performed (Figure 3). In cases of unresectable tumors and BRAF V600E mutation, dabrafenib + trametinib, and in unresectable tumors with BRAF (wildtype), EBRT or palliative care is indicated. Dabrafenib and trametinib are prescribed preferentially as a combination, also in non-small cell lung cancer and melanoma. In one trial, where patients with de-differentiated TC might also have been included, a response rate of 70% has been reported. These TKI may cause adverse effects, such as a lack of appetite, rash, and hypertension. Severe pyrexia upon therapy with trametinib is particularly serious and difficult to treat and may warrant discontinuation of the drug.
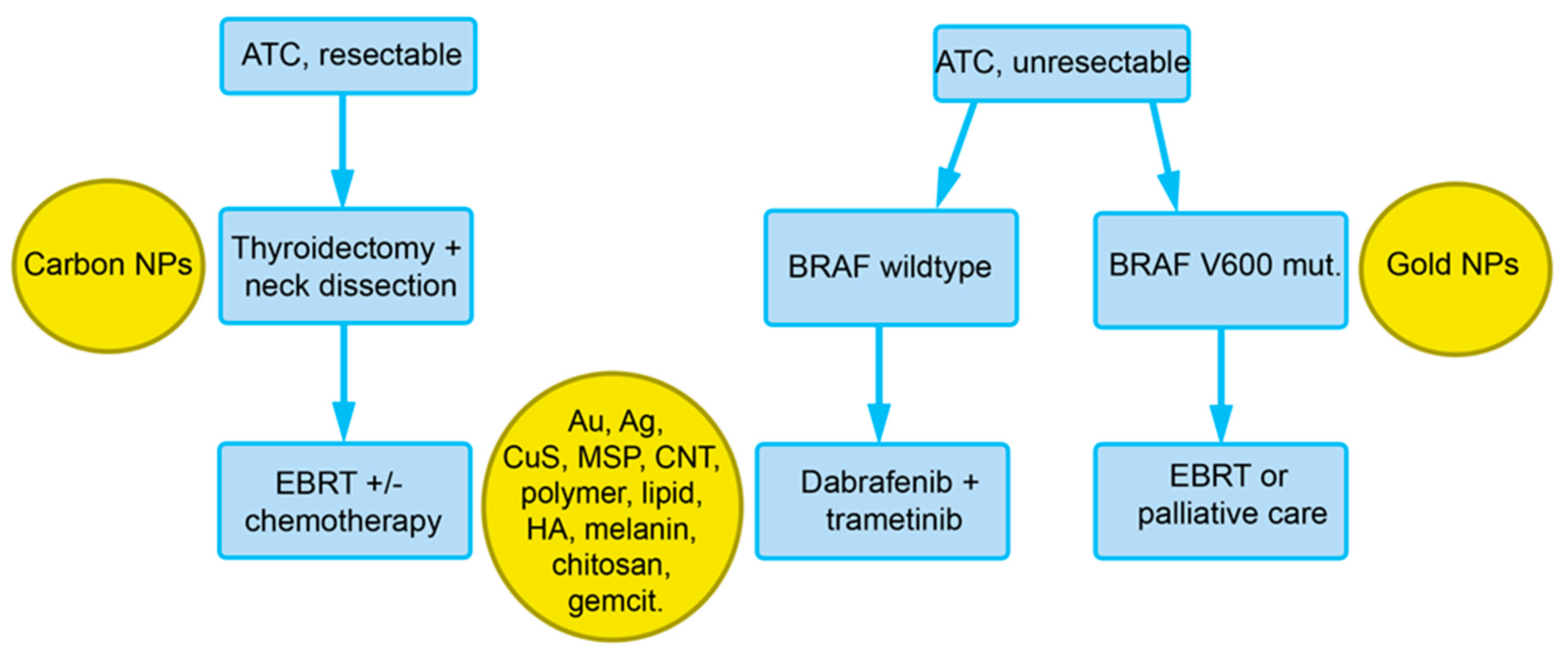
Figure 3. Overview of the recommendations of the European Society for Medical Oncology (ESMO) for the treatment of ATC with indications for nanoparticles that could be used at the respective diagnostic/treatment phase (see Section 4). Abbreviations: Ag, silver; Au, gold; BRAF V600E, V-raf murine sarcoma viral oncogene homolog B; CNT, carbon nanotubes; EBRT, external beam radiation therapy; HA, hyaluronic acid; gemcit, gemcitabine; MSP, mesoporous silica particles.
The TKIs playing an established role in the treatment of TC act on RET, FGFR, cKIT, PDGFR, VEGFR (lenvantinib), BRAF, RET, c-KIT, PDGFR, VEGFR (sorafenib), BRAF (dabrafenib), MEK (trametinib), RET, VEGFR (cabozantinib), and RET, EGFR, and VEGFR (vandetanib).
3. Nanoparticles in the Diagnosis of TC
Biomarkers can help in diagnosis or for disease monitoring. Their use in monitoring of TC is more important because the search for specific biomarkers so far has not been successful. Markers related to metabolism, thyroid function, and tumor phenotype have been identified but the majority were general cancer markers. BRAF V600 and calcitonin levels have a role in the treatment strategy (Figure 1) but for other hormones a lack of clear cut-off values presented the greatest problem. Recently, the first markers for ATC have been reported. Melanoma-associated antigen A3 (MAGEA3) and the oncofetal IGF2 mRNA binding protein 1 (IGF2BP1) were suitable for differentiation between ATC and undifferentiated PTC and IGF2BP1 may have the potential to serve as a prognostic marker.
3.1. Circulating Biomarkers
Liquid biopsies are useful tools to detect biomarkers, such as circulating tumor cells, circulating vesicles (extracellular vesicles), circulating nucleic acid, and circulating proteins derived from tumor cells. Gold NPs are the most often used NPs because they offer detection by fluorescence, colorimetry, photoacoustics, surface enhanced Raman scattering (SERS), electrochemistry, dynamic light scattering (DLS), or localized surface plasmon resonance (LSPR). Better performance regarding lower limits of detection have been reached for TSH, Tg and calcitonin has been reached. Gold NP-based assays for detection of BRAF V600 mutations have been established. Fast translation into clinical practice, however, is hindered by less common protocols and platforms.
Fine Needle Aspiration Biopsy is the gold standard for evaluation of suspicious thyroid lesions, although differentiation between FTC and follicular adenoma or between oncocytic thyroid adenoma and Hürthle cell carcinoma pose problems. Nanotechnology may provide an additional tool by proteomic analysis of the patient-specific protein corona on gold, silver, and iron NPs. The protocol uses formalin-fixed, paraffin-embedded samples for extraction of the protein, coating of the NPs, separation of the proteins by electrophoresis, digestion of the proteins in the gel, and identification by mass spectrometry.
3.2. In Vivo Imaging and Combination of Imaging and Therapeutics (Theranostics)
Common technologies in diagnosis, staging, and management of TC include ultrasound, CT, and MRI. The role of FDG-PET is less clear. 131/123I whole body scans can be used to detect residual locoregional disease in the neck and distant metastases. It is ideally combined with 3D conventional imaging using single photon emission computed tomography (SPECT) in combination with CT. [18F]FDOPA and [68Ga]DOTATOC are the preferred tracers for MTC. Primary TC is located near the body surface and detection may profit from specific techniques less suitable for deeply located tumors due to the low penetration depths of fluorescent light and ultrasound. Photothermal treatment, which relies on the absorption of NIR light and conversion to thermal energy, can be effective to a depth of 2–3 cm below the skin. The penetration depths of ultrasound are difficult to indicate because absorption is tissue specific (e.g., low absorption in blood and fat), and the thicknesses of the tissue layers in individual patients are unknown. The maximum depths to achieve effective heating at a 2.5 MHz frequency of unfocused ultrasound have been indicated as 3–6 cm. Low-intensity focused ultrasound (LIFUS) can be used to combine imaging and treatment of superficially located tumors. The technology consists of sonodynamic therapy, ultrasound-mediated chemotherapy, ultrasound-mediated gene delivery, and anti-vascular ultrasound therapy.
The future of NPs in diagnosis is tightly linked to the availability of specific TC tumor markers. If such markers are identified, there will be the possibility to optimize their detection. The option to identify new markers by use of the patient-specific protein corona may present a promising yet not very fast tool to improve diagnosis of TC.
4. Nanoparticles in the Treatment of TC
Overall, inorganic and organic NPs have been studied in TC. Gold NPs and mesoporous (hybrid) silica NPs were reported to be suitable for delivery of conventional drugs and proteins. Gold particles may be also used for photothermal therapy. Targeting strategies range from general principles in cancer treatment (pH-dependency, overexpression of transferrin or VEGF receptor) to more TC-specific targets, such as the TSH receptor. The latter strategy has the disadvantage that de-differentiated TC usually do not express the receptor any more. Liposomes and polymeric NPs were used for delivery of conventional drugs, re-differentiating compounds, and siRNA. Active and passive targeting was effective.
Inorganic and organic NPs possess different advantages and limitations. The inorganic NPs, e.g., gold NPs, enable the use of additional technologies but could pose problems, for example, regarding accumulation in the human body. Organic NPs, typically lipid nanoparticles, are faster degraded and removed from the body. They offer the possibility to load a broad variety of payloads but the option to use additional anti-tumor strategies (e.g., photodynamic therapy and hyperthermia) is more limited. Reproducible, large-scale synthesis represents a challenge for all NP-based treatments. More information on nanomedicine in cancer is available in reviews on this topic.
Despite the various proposed uses of NPs in diagnosis and treatment of TC, data from clinical trials are limited.
CALAA-01 is a transferrin-targeted nanocomplex consisting of siRNA to reduce expression of the M2 subunit of ribonucleotide reductase and cyclodextrin-containing polymer. The trial was terminated due to dose-limiting toxicities (e.g., grade 3 ischemic colitis, grade 2 diarrhea, and grade 1 fever, as well as grade 4 fatigue, grade 2 flu-like symptoms, grade 2 muscular spasm, and grade 1 nausea).
Future and ongoing trials will evaluate the use of several nanoparticles as a tracer for lymph node mapping to improve the surgical treatment of thyroid cancer. These particles include carbon nanoparticles alone, carbon nanoparticles in combination with indocyanine green dye, and anti-Tg antibody functionalized fluorescent polystyrene particles.
For multidrug chemotherapy of TC, the efficacy of the approved albumin-bound particle form of paclitaxel (nab-paclitaxel, Abraxane®) is being evaluated in combination with the anti-PDL-1 antibody Atezolizumab (NCT03181100, phase II) and in combination with the glycogen synthase kinase 3 beta (GSK-3β) inhibitor, 9-ING-41 (NCT03678883, phase I/II).
5. Conclusions
Compared to other tumors, a relatively low number of NPs has been studied for diagnosis and treatment of TC. Reasons for that may be that TC is not a common cancer and TC, with the exception of de-differentiated tumors and ATC, is not highly aggressive. Tumor accumulation of NPs can either use passive targeting by the EPR effect or use tumor-specific targeting molecules. NPs could improve diagnosis and monitoring of TC by more sensitive sensor technologies, e.g., BRAF V600 mutation and for imaging. Encapsulation into nanocarriers increased the efficacy of doxorubicin, sorafenib, and gemcitabine treatment, and decreased their adverse effects. Improved delivery of retinoic acid to TC cells could improve the re-differentiation therapy of de-differentiated TC.
All NPs, except carbon NPs during TC surgery, are in the pre-clinical phase of drug development and, quite frequently, only in vitro cellular studies, often without comparison to normal cells, are available. Although encouraging results from animal studies have been reported, their value for predicting efficacy in humans is subject to the inherent limitations of the models. These include the clonal origin of the xenografts, leading to lower heterogeneity, the larger relative size of the xenografts compared to human tumors but smaller size at treatment, immunodeficient status of the athymic mice, inoculation at a young age, subcutaneous versus orthotopic localization, and often the absence of metastases in the mouse models. These differences may not be very relevant because rapid growth and high vascularization are also seen in human ATC. Further, metastatic disease can be mimicked by the generation of orthotopic tumors using injection of the appropriate cells (e.g., 8505C) into the thyroid. However, human tumors still differ from the xenografts by their smaller sizes relative to body weight, and the older age of the host. The benefit of using carbon NPs for improved TC surgery is still not clear. Specific technologies less suitable for the treatment of deeply located cancers have some potential for unresectable ATC, namely LIFUS and phototherapy using NIR irradiation.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13164063
