What is called an ionic liquid (IL) has a very broad definition, comprising multiple substances possessing a wide diversity of structures and properties. An IL consists of both organic and inorganic ions, and may contain more than one cation or anion. Normally, a substance is considered to be an IL if completely composed of ions, with a melting point below 100 °C. Ionic liquids containing lanthanides or lanthanide compounds in ionic liquids are very important in the field of soft luminescent materials.
- lanthanides
- ionic liquids
- luminescence
- spectroscopy
- NIR
1. Introduction
Within ILs, there are electrostatic and dispersive interactions at different length scales, leading to a highly anisotropic character. The ions have a large structural diversity which varies from inorganic to organic, simple to complex, including fully or partially ionized acid or base, organic polymeric metal ions, or metalated coordination polymers [1], giving a boundless variety of cation/anion combinations, estimated around the order of 1019 [2].
2. Luminescent Ln-Ionic Liquids beyond Europium
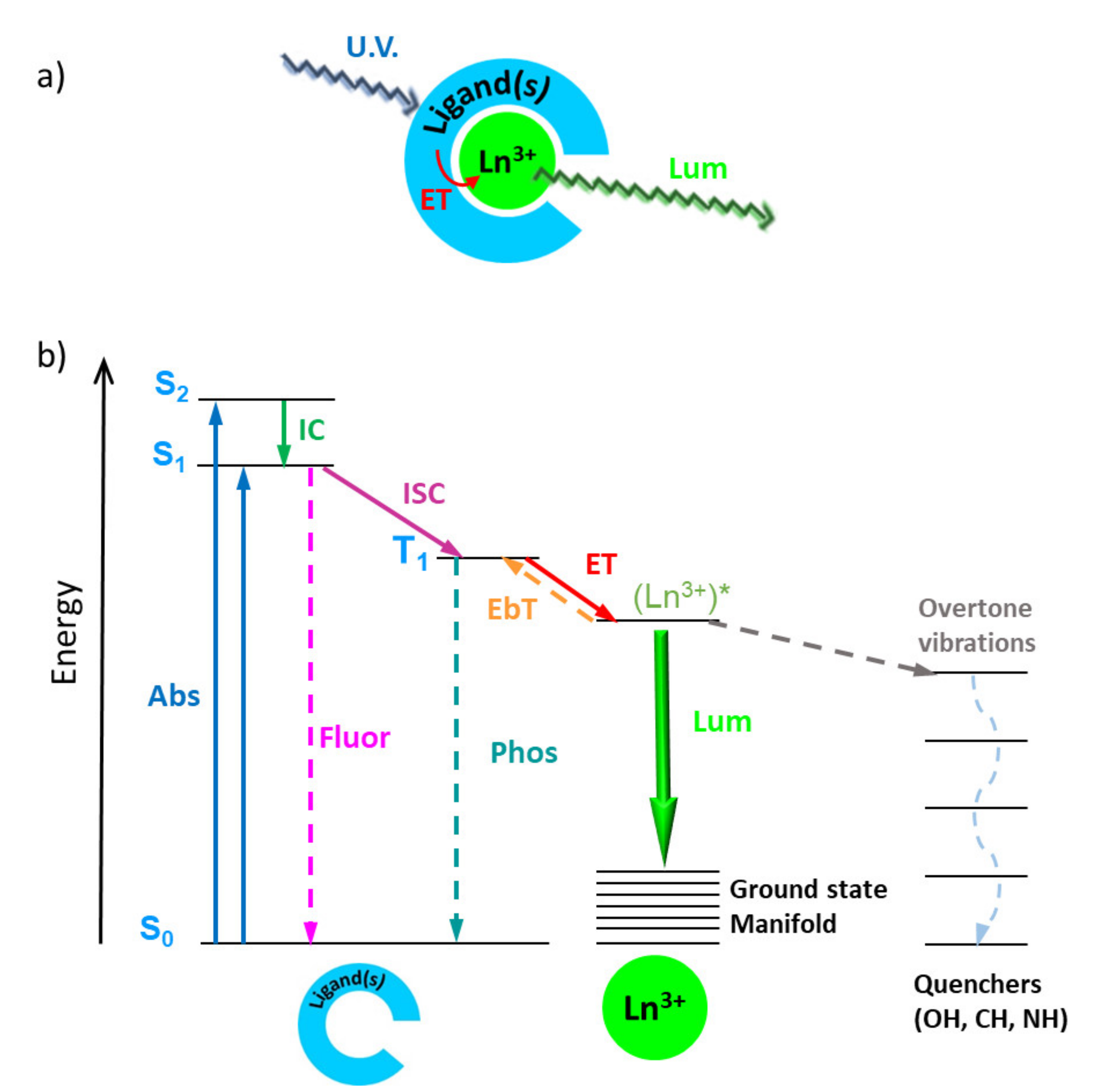
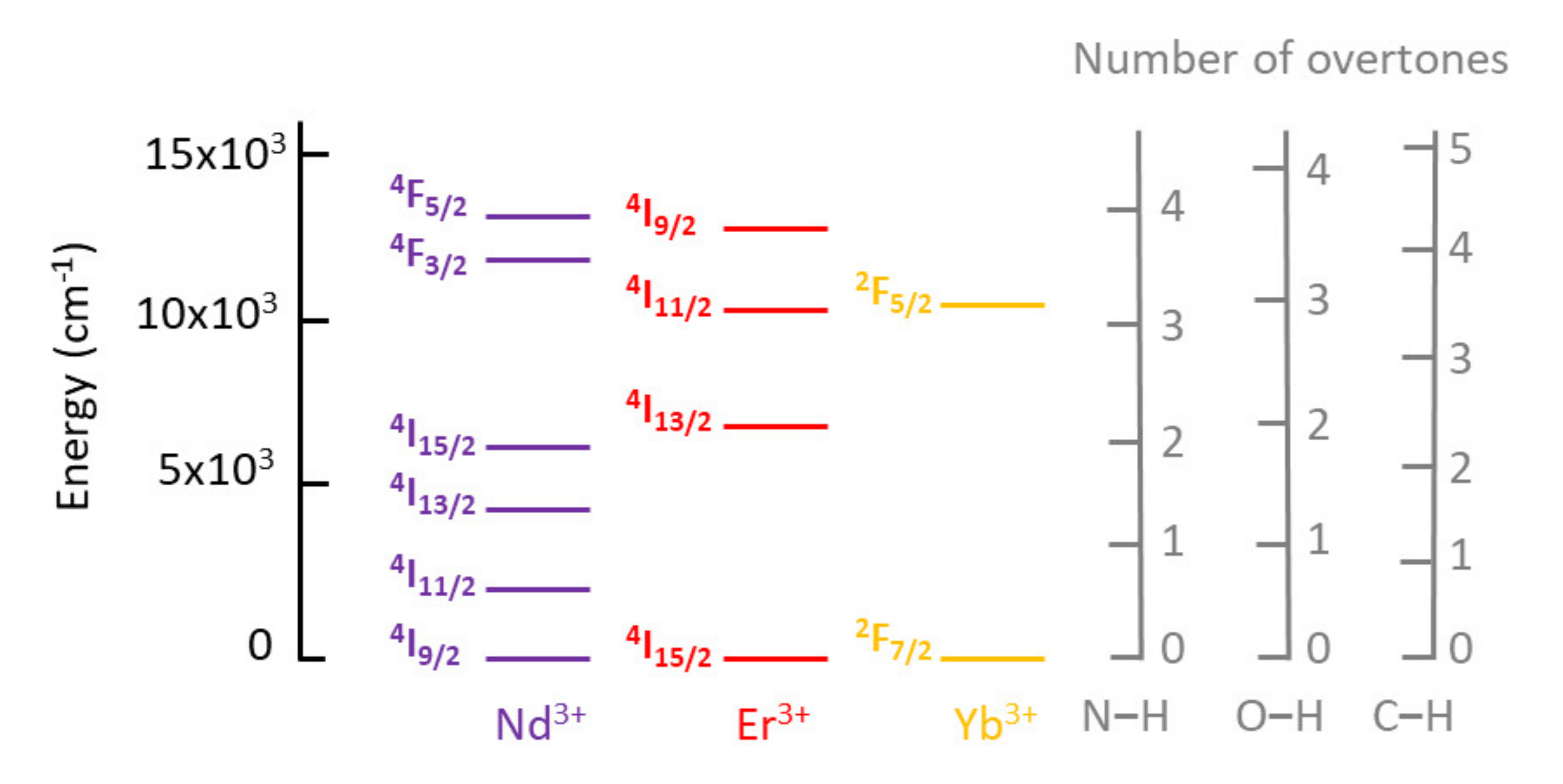
To obtain highly efficient Ln molecular light-conversion devices, it is necessary to optimize several parameters: avoid self-quenching channels, use chromophores with high molar absorbance and ideal energy positions of singlet and triplet states (for an efficient energy transfer to Ln3+ ions), while avoiding competitive non-radiative pathways such as multiphonon relaxation to high-energy vibrations (e.g., O–H, C–H, and N–H stretching modes). With this context in mind, the combination of lanthanides and ionic liquids began by using ILs as matrices to protect Ln3+ ions from vibration-induced deactivation processes—mainly from the ever-present water adsorbed in organic solvents. Although ILs proved to provide good protection against the presence of water within the first and/second coordination spheres of the Ln centers, due to the low solubility of the Ln salts, this method usually enabled low concentrations of Ln ions, although higher concentrations could be achieved by the use of the same anionic moieties as both the ligand (of the Ln complex) and the anion (of the ILs). This shortcoming was circumvented by preparing Ln-based ionic liquids, either via direct preparation by metathesis, or by dissolving Ln salts in ILs with anions with coordinating capabilities. Ln-containing ionic liquids proved to be promising materials because, although liquids, they provide a low-phonon environment for the Ln3+ center, leading to appreciable excitation state lifetimes. Typically, liquid-state lanthanide compounds present lower emission quantum yields (Φ) than those in the solid state, due to a less rigid environment and energy loss from collisions. As such, it is not surprising that emission quantum yields for Ln@ILs are very low. The same reasoning is applicable to Ln-ILs although, surprisingly, out of the 58 Ln-ILs presented in our recent review paper (see reference at the end of this entry), only one RTIL—[C6mim]3[Sm(NO3)6]—had its emission quantum yield determined, with a value of 2.73% [38]. Another important aspect to stress is that since Ln@ILs are liquids, no structural characterization was available for the majority of these compounds.
It is worth mentioning that many of the Ln-ILs were studied not only as phosphors, but also as paramagnetic liquids, opening avenues for multifunctional applications. Additionally, the combination of Ln and ILs has aroused so much interest that it led to the emergence of a new field of research, focused specifically on soft materials. In this area, new ionogels have been developed through covalently grafting—or simply dispersing—Ln complexes into silica-based materials, polymer matrices, liquid crystals, etc.
It was not intended to include ionogels in this entry but, since they are becaming more and more importent and just as an example, a simple and environmentally friendly (solvent-free) preparation of ionogels via the incorporation of Ln-ILs within poly(methyl methacrylate) (PMMA) was reported by Wang et al. as early as 2013 [39]. In that work, the ILs Tb(sal)@[Carb-mim][Tf2N] and Eu(tta)@[Carb-mim] [Tf2N] were directly dissolved into MMA monomers with azodiisobutyronitrile (polymerization initiator), with stirring at 80 °C, yielding a yellowish liquid that was then cast into glass slides or glass bottles. After drying, ionogels in the form of monoliths, films, and flexible self-standing films could be obtained ( Figure 14 ).
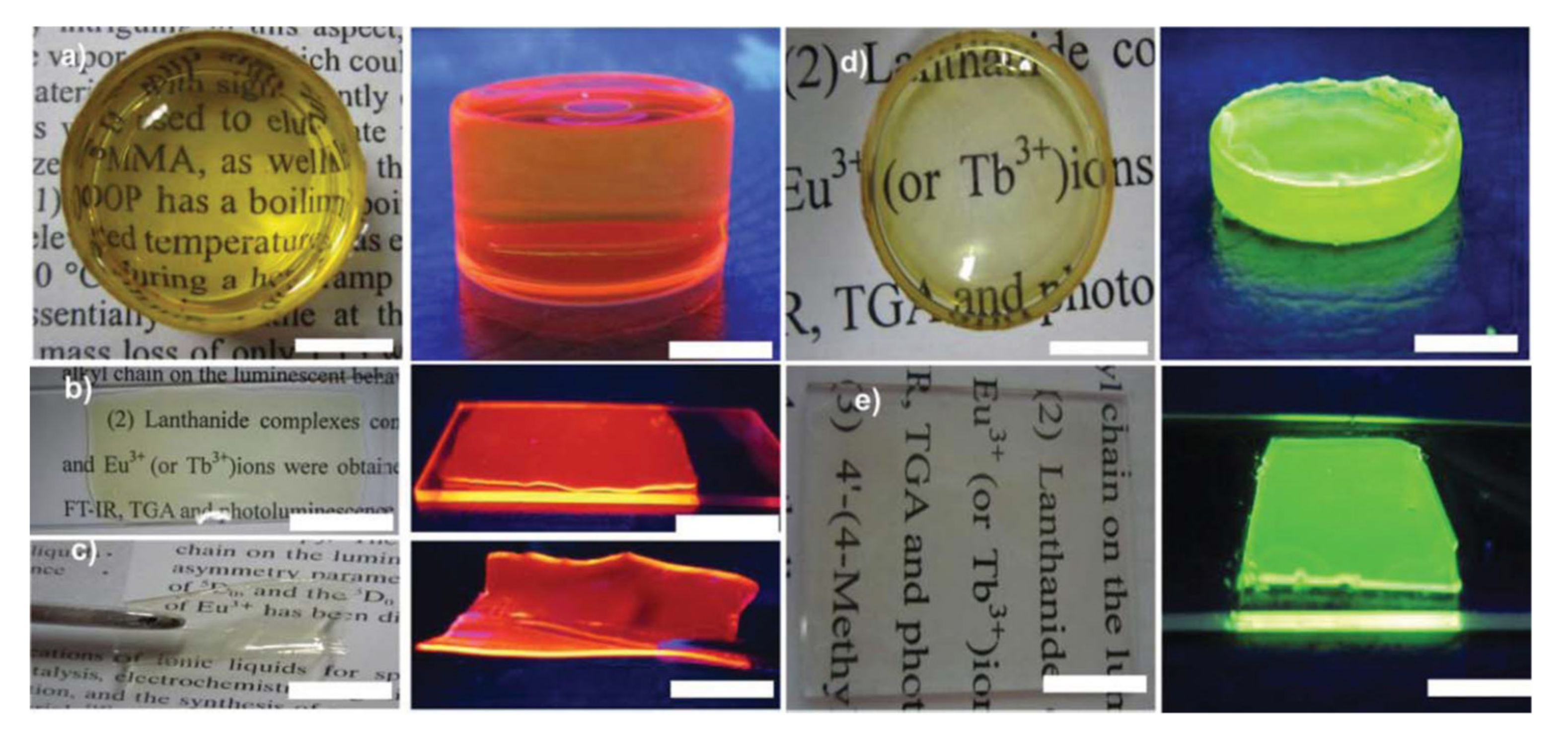
Figure 14. Digital photos of the Ln-ILs-PMMA: (a–c) Eu(tta)–[Carb-mim][Tf2N]@PMMA under daylight (right) and UV light (left); (d–e) Tb(sal)–[Carb-mim][Tf2N]@PMMA under daylight (right) and UV light (left). The scale bar is 1.0 cm. Reproduced with permission from [39].
The accomplishments described in this entry prove Ln-ILs to be outstanding and promising optical materials. However, this field of research is still underdeveloped when compared with other fields of ionic liquid chemistry. Therefore, new studies focusing on different combinations of Ln ions and new ligands will certainly lead to more efficient luminescent molecular devices, paving the way for practical applications as varied as catalysis, biochemical analysis, energy production, and non-invasive diagnostics, such as biolabels.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26164834
References
- Lei, Z.-G.; Chen, B.-H.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635.
- Prodius, D.; Smetana, V.; Steinberg, S.; Wilk-Kozubek, M.; Mudryk, Y.; Pecharsky, V.K.; Mudring, A.-V. Breaking the paradigm: Record quindecim charged magnetic ionic liquids. Mater. Horiz. 2017, 4, 217–221.
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083.
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80.
- Giernoth, R. Task-Specific Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839.
- Zhao, H.; Xia, S.Q.; Ma, P.S. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096.
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629.
- Berthod, A.; Ruiz-Angel, M.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18.
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end-of-life electronic wastes-a review. Crit. Rev. Env. Sci. Tech. 2019, 49, 212–275.
- Sun, X.; Luo, H.; Dai, S. Mechanistic investigation of solvent extraction based on anion-functionalized ionic liquids for selective separation of rare-earth ions. Dalton Trans. 2013, 42, 8270–8275.
- Maria, L.; Cruz, A.; Carretas, J.M.; Monteiro, B.; Galinha, C.; Gomes, S.S.; Araujo, M.F.; Paiva, I.; Marcalo, J.; Leal, J.P. Improving the selective extraction of lanthanides by using functionalised ionic liquids. Sep. Purif. Technol. 2020, 237, 116354.
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of ionic liquids in hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343.
- Bermudez, M.D.; Jimenez, A.E.; Sanes, J.; Carrion, F.J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908.
- Rooney, D.; Jacquemin, J.; Gardas, R. Thermophysical Properties of Ionic Liquids. In Ionic Liquids. Topics in Current Chemistry; Kirchner, B., Ed.; Springer: Berlin, Germany, 2009; Volume 290, pp. 185–212.
- Mudring, A.-V. Optical Spectroscopy and Ionic Liquids. In Ionic Liquids. Topics in Current Chemistry; Kirchner, B., Ed.; Springer: Berlin, Germany, 2009; Volume 290, pp. 285–310.
- Tang, S.-F.; Mudring, A.-V. Highly Luminescent Ionic Liquids Based on Complex Lanthanide Saccharinates. Inorg. Chem. 2019, 58, 11569–11578.
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 1–263.
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077.
- Lei, N.; Shen, D.; Wanga, J.; Chen, X. Flexible and enhanced multicolor-emitting films co-assembled by lanthanide complexes and a polymerizable surfactant in aqueous solution. Soft Matter 2018, 14, 9143–9152.
- Prodius, D.; Mudring, A.-V. Rare earth metal-containing ionic liquids. Coord. Chem. Rev. 2018, 363, 1–16.
- Weissman, S.I. Intramolecular Energy Transfer the Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217.
- Bünzli., J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47.
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord. Chem. Rev. 2019, 399, 213028.
- Varaksina, E.A.; Taydakov, I.V.; Ambrozevich, S.A.; Selyukov, A.S.; Lyssenko, K.A.; Jesus, L.T.; Freire, R.O. Influence of fluorinated chain length on luminescent properties of Eu3+ β-diketonate complexes. J. Lumin. 2018, 196, 161–168.
- Driesen, K.; Nockemann, P.; Binnemans, K. Ionic liquids as solvents for near-infrared emitting lanthanide complexes. Chem. Phys. Lett. 2004, 395, 306–310.
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227.
- Mudring, A.-V.; Tang, S. Ionic Liquids for Lanthanide and Actinide Chemistry. Eur. J. Inorg. Chem. 2010, 2569–2581.
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410.
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018.
- Cheng, K.-L.; Yuan, W.-L.; He, L.; Tang, N.; Jian, H.-M.; Zhao, Y.; Qin, S.; Tao, G.-H. Fluorescigenic Magnetofluids Based on Gadolinium, Terbium, and Dysprosium-Containing Imidazolium Salts. Inorg. Chem. 2018, 57, 6376–6390.
- Ramos, T.J.S.; Berton, G.H.; Júnior, S.A.; Cassol, T.M. Photostable soft materials with tunable emission based on sultone functionalized ionic liquid and lanthanides ions. J. Lumin. 2019, 209, 208–216.
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45.
- Tang, S.; Babai, A.; Mudring, A.-V. Europium-based ionic liquids as luminescent soft materials. Angew. Chem. Int. Ed. 2008, 47, 7631–7634.
- Puntus, L.N.; Pekareva, I.S.; Lyssenko, K.A.; Shaplov, A.S.; Lozinskaya, E.I.; Zdvizhkov, A.T.; Buzin, M.I.; Vygodskii, Y.S. Influence of ionic liquid anion nature on the properties of Eu-containing luminescent materials. Opt. Mater. 2010, 32, 707–710.
- Bruno, S.M.; Ferreira, R.A.S.; Paz, F.A.A.; Carlos, L.D.; Pillinger, M.; Ribeiro-Claro, P.; Gonçalves, I.S. Structural and Photoluminescence Studies of a Europium(III) Tetrakis (beta-diketonate) Complex with Tetrabutylammonium, Imidazolium, Pyridinium and Silica-Supported Imidazolium Counterions. Inorg. Chem. 2009, 48, 4882–4895.
- Guillet, E.; Imbert, D.; Scopelliti, R.; Bünzli, J.-C.G. Tuning the emission color of europium-containing ionic liquid-crystalline phases. Chem. Mater. 2004, 16, 4063–4070.
- Nockemann, P.; Beurer, E.; Driesen, K.; Van Deun, R.; Van Hecke, K.; Van Meervelt, L.; Binnemans, K. Photostability of a highly luminescent europium beta-diketonate complex in imidazolium ionic liquids. Chem. Commun. 2005, 34, 4354–4356.
- Tang, N.; Zhao, Y.; He, L.; Yuan, W.-L.; Tao, G.-H. Long-lived luminescent soft materials of hexanitratosamarate(III) complexes with orange visible emission. Dalton Trans. 2015, 44, 8816–8823.
- Wang, H.; Wang, Y.; Zhang, L.; Li, H. Transparent and luminescent ionogels based on lanthanide-containing ionic liquids and poly(methylmethacrylate) prepared through an environmentally friendly method. RSC Adv. 2013, 3, 8535–8540.
