Atrial Fibrillation (AF) and Heart Failure (HF) are closely linked to each other, as each can be either the cause of or the result of the other. Successfully treating one of the two entities means laying the basis for treating the other one as well. AF is the most common cardiac arrhythmia, It is predisposed by several risk factors such as HF, ischemic heart disease, high blood pressure, valvular heart disease, sleep apnea, and diabetes, and at the same time increases the risk of developing heart failure of any kind (heart failure with preserved ejection fraction, HFpEF; heart failure with mid-range ejection fraction, HFmrEF; heart failure with reduced ejection fraction, HFrEF). AF and heart failure co-exist in up to 30% of patients and are closely linked to each other, as each can be either the cause of or the result of the other (“Atrial Fibrillation Begets Heart Failure and Vice Versa”). When both conditions occur in the same patient, the prognosis is worse than with either condition alone. Pulmonary Vein Isolation (PVI) is a well established treatment option in patients with symptomatic atrial fibrillation. Studies investigating PVI in patients with AF and HF will be discussed in this paper.
- catheter ablation
- heart failure
- atrial fibrillation
1. Introduction
2. Pathophysiologic and Cellular Mechanisms
3. Scientific Evidence Concerning Rate vs. Rhythm Control
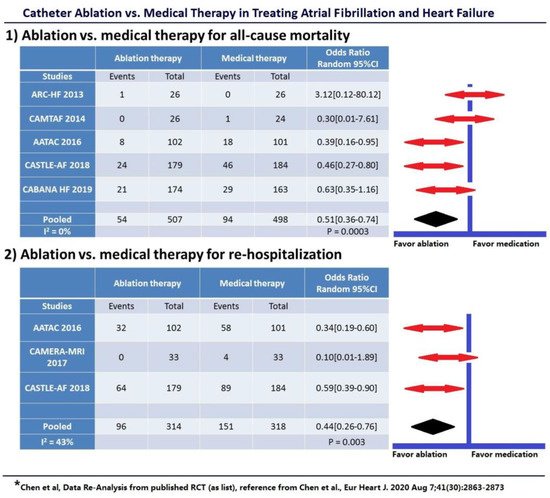
4. Scientific Evidence Concerning AF Ablation in HF Patients
5. Recommendations on AF Treatment in HF Patients
| Guideline | Recommendation | Class | Level of Evidence |
|---|---|---|---|
| 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) |
AF catheter ablation is recommended to reverse LV dysfunction in AF patients when tachycardia-induced cardiomyopathy is highly probable, independent of their symptom status | I | B |
| AF catheter ablation should be considered in selected AF patients with HF with reduced LVEF to improve survival and reduce HF hospitalization | IIa | B | |
| 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation |
AF catheter ablation may be reasonable in selected patients with symptomatic AF and HF with reduced left ventricular (LV) ejection fraction (HFrEF) to potentially lower mortality rate and reduce hospitalization for HF | IIb | B-R |
| 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensusstatement on catheter and surgical ablation of atrial fibrillation |
It is reasonable to use similar indications for AF ablation in selected patients with heart failure as in patients without heart failure. | IIa | B-R |
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jcm10163512
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847.
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.; Cheng, S.; Vasan, R.S.; Lee, D.; et al. Response to Letter Regarding Article, “Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction”. Circulation 2016, 133, 484–492.
- McManus, D.D.; Hsu, G.; Sung, S.H.; Saczynski, J.S.; Smith, D.H.; Magid, D.J.; Gurwitz, J.H.; Goldberg, R.J.; Go, A.S. Cardiovascular Research Network PRESERVE Study. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J. Am. Heart Assoc. 2013, 2, e005694.
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925.
- Piccini, J.P.; Hammill, B.G.; Sinner, M.F.; Hernandez, A.F.; Walkey, A.; Benjamin, E.; Curtis, L.H.; Heckbert, S.R. Clinical course of atrial fibrillation in older adults: The importance of cardiovascular events beyond stroke. Eur. Hear J. 2013, 35, 250–256.
- Shinbane, J.S.; Wood, A.M.; Jensen, D.; Ellenbogen, A.K.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-Induced Cardiomyopathy: A Review of Animal Models and Clinical Studies. J. Am. Coll. Cardiol. 1997, 29, 709–715.
- Spinale, F.G.; Tomita, M.; Zellner, J.L.; Cook, J.C.; Crawford, F.A.; Zile, M. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am. J. Physiol. Circ. Physiol. 1991, 261, H308–H318.
- Tomita, M.; Spinale, F.G.; Crawford, F.A.; Zile, M.R. Changes in left ventricular volume, mass, and function during the development and regression of supraventricular tachycardia-induced cardiomyopathy. Disparity between recovery of systolic versus diastolic function. Circulation 1991, 83, 635–644.
- Wasmund, S.L.; Li, J.-M.; Page, R.L.; Joglar, J.A.; Kowal, R.C.; Smith, M.L.; Hamdan, M.H. Effect of Atrial Fibrillation and an Irregular Ventricular Response on Sympathetic Nerve Activity in Human Subjects. Circulation 2003, 107, 2011–2015.
- Marx, O.S.; Reiken, S.; Hisamatsu, Y.; Jayaraman, T.; Burkhoff, D.; Rosemblit, N.; Marks, A.R. PKA Phosphorylation Dissociates FKBP12.6 from the Calcium Release Channel (Ryanodine Receptor): Defective Regulation in Failing Hearts. Cell 2000, 101, 365–376.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498.
- Van Gelder, I.C.; Groenveld, H.F.; Crijns, H.J.; Tuininga, Y.S.; Tijssen, J.G.; Alings, A.M.; Hillege, H.L.; Bergsma-Kadijk, J.A.; Cornel, J.H.; Kamp, O.; et al. RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010, 362, 1363–1373.
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833.
- Freemantle, N.; Lafuente-Lafuente, C.; Mitchell, S.; Eckert, L.; Reynolds, M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011, 13, 329–345.
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.; Buxton, A.E.; Camm, A.J.; et al. Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008, 358, 2667–2677.
- Khan, M.N.; Jaïs, P.; Cummings, J.; Di Biase, L.; Sanders, P.; Martin, D.O.; Kautzner, J.; Hao, S.; Themistoclakis, S.; Fanelli, R.; et al. Pulmonary-Vein Isolation for Atrial Fibrillation in Patients with Heart Failure. N. Engl. J. Med. 2008, 359, 1778–1785.
- Hunter, R.J.; Berriman, T.J.; Diab, I.; Kamdar, R.; Richmond, L.; Baker, V.; Goromonzi, F.; Sawhney, V.; Duncan, E.; Page, S.P.; et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ. Arrhythm. Electrophysiol. 2014, 7, 31–38.
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Trivedi, C.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistoclakis, S.; Dello Russo, A.; et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. CASTLE-AF Investigators. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427.
- Kuck, K.H.; Merkely, B.; Zahn, R.; Arentz, T.; Seidl, K.; Schlüter, M.; Tilz, R.R.; Piorkowski, C.; Gellér, L.; Kleemann, T.; et al. Catheter Ablation Versus Best Medical Therapy in Patients with Persistent Atrial Fibrillation and Congestive Heart Failure: The Randomized AMICA Trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007731.
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Moretz, K.; Poole, J.E.; Mascette, A.; Rosenberg, Y.; Jeffries, N.; et al. CABANA Investigators. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am. Heart J. 2018, 199, 192–199.
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B.; et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021, 143, 1377–1390.
- Samuel, M.; Abrahamowicz, M.; Joza, J.; Beauchamp, M.-E.; Essebag, V.; Pilote, L. Long-term effectiveness of catheter ablation in patients with atrial fibrillation and heart failure. Europace 2020, 22, 739–747.
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. EAST-AFNET 4 Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316.
- Chen, S.; Pürerfellner, H.; Meyer, C.; Acou, W.-J.; Schratter, A.; Ling, Z.; Liu, S.; Yin, Y.; Martinek, M.; Kiuchi, M.G.; et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: A stratified pooled analysis of randomized data. Eur. Hear J. 2019, 41, 2863–2873.
- Gopinathannair, R.; Chen, L.Y.; Chung, M.K.; Cornwell, W.K.; Furie, K.L.; Lakkireddy, D.R.; Marrouche, N.F.; Natale, A.; Olshansky, B.; Joglar, J.A. Managing Atrial Fibrillation in Patients with Heart Failure and Reduced Ejection Fraction: A Scientific Statement from the American Heart Association. Circ. Arrhythmia Electrophysiol. 2021, 14, e000078.
- Packer, D.L.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Poole, J.P.; Bahnson, T.D.; Mark, D.B.; Lee, K.L. Ablation of Atrial Fibrillation in Heart Failure Patients: Additional outcomes of the CABANATrial. Heart Rhythm 2019, 16, S35.
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. Document Reviewers: 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160.
- Writing Group Members; January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019, 16, e66–e93.
