4-Hexylresorcinol (4HR) is a synthetic resorcinolic lipid that has been used as an anti-parasitic and antiseptic agent since the 1920s.
1. Introduction
Growth and development are largely controlled by genetic regulation. However, twins who share identical genomes develop remarkable differences in their genomic distribution over time [
1]. This means that both genetic and environmental regulations affect gene expression and its activity, and epigenetics could be a major explanation for this phenomenon. Epigenetics means structural chromatin changes that do not involve alterations in the DNA sequence. This includes chromatin remodeling, DNA methylation, histone modification, and micro-RNAs. Of the mechanisms of epigenetic regulations, histone protein modification is the mechanism that induces gene expression and suppression by post-translational modification via acetylation, methylation, and phosphorylation in the histone N-terminal region of the nucleosome [
2].
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from histones and suppress gene expression by allowing for histones to bind more tightly with DNA [
3]. They can perform as erasers of epigenetic marks on chromatin, counterbalanced by histone or lysine acetyltransferases (HAT/KAT), which add acetyl groups to lysines and allow for gene expression [
3]. In humans, there are 18 HDAC genes that are categorized into four families (Classes I–IV) on the basis of their subcellular location, structure, and function [
3]. Classes I (HDACs 1–3 and 8), IIa (HDACs 4, 5, 7, and 9), IIb (HDACs 6 and 10), and IV (HDAC11) are zinc-dependent HDACs. Class III comprises the structurally and mechanistically distinct NAD+-dependent enzyme [
4].
Bone development takes place via two main processes during the early stages of embryonic development: intramembranous and endochondral bone formation. Once bone is developed, it is remodeled throughout life by the tight regulation of bone resorption and new bone formation. These whole processes require harmonized orchestration between the expression of specific key genes with transcription factors and cofactors [
5]. HDACs contribute to these different steps of bone development by the deacetylation of transcription factors [
6]. From studies using knockout mice, HDAC1-null embryos showed severe defects in head formation and died before endochondral ossification began [
7]. HDAC2 mutant mice were reported to develop reduced body size [
8]. HDAC4-deficient mice have premature mineralization of skeletal elements that prevent longitudinal growth [
9]. HDAC8 knockout mice resulted in impairment of skull development [
10]. These results indicate that HDACs extensively and intimately affect skeletal development and bone maintenance.
Histone deacetylase inhibitors (HDACi) are small compounds with a moiety that can integrate into zinc-containing catalytic sites of Class I and II HDACs with a capping group and linker between them. They can be classified as short-chain fatty acids (e.g., valproate and sodium butyrate), hydroxamic acids (depsipeptide, FR901228), cyclic peptides (MS-275), enzamide hydroxamic acids (SAHA, trichostatin A (TSA)), epoxyketones (trapoxin), and hybrid molecules (CHAP31 and CHAP50) according to their basic structure [
11]. HDACi can upregulate gene expression by inhibiting the enzyme activity of HDAC and is used as a treatment for various diseases such as cancer, osteoporosis, arthritis, epilepsy, and mood disorder [
4]. Valproic acid, sodium butyrate, and TSA are pan-HDACi that can suppress different classes of HDAC [
11]. On the other hand, romidespin and entinostat (MS-275) are Class I selective HDACi [
11].
Many of the studies about the effects of HDACi on skeletal development were conducted on individual bone cells and indicated the promising possibility of enhancing osteogenesis and decreasing osteoclast activity [
12,
13,
14,
15]. However, several clinical studies reported that the long-term administration of valproate decreased bone mineral density in children and adults [
16,
17,
18], causing increased fracture risk [
19]. These conflicts between in vitro and in vivo studies require further research to find the exact mechanism of how HDACi affects skeletal bone development.
2. 4HR Administration Inhibits Class I HDAC Activity and Increases the Acetylation Level of Cellular Proteins
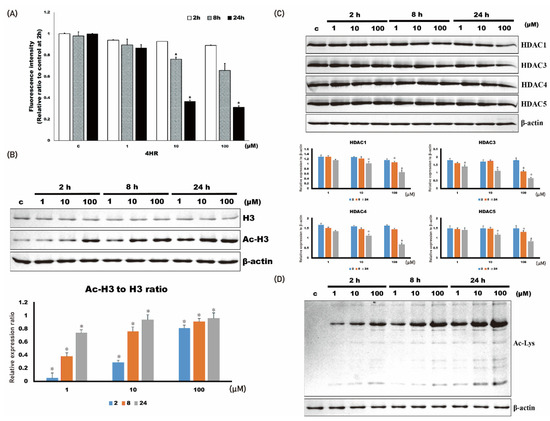
The activity of HDAC was decreased by the administration of 4HR (1–100 μM; Figure 1A). When HDAC activity was compared among groups, its activity in Saos-2 cells treated with 4HR (1 μM–100 μM) decreased significantly at 8 and 24 h (p < 0.001 and 0.009, respectively). In the post hoc test, 10 and 100 μM 4HR treatments resulted in significantly lower values compared with those of the untreated control group at 8 h (p = 0.003 and <0.001, respectively). When observed at 24 h after 4HR treatments, 100 μM 4HR treatments resulted in significantly lower values compared with those of the untreated control group (p = 0.009).
Figure 1. 4HR administration and histone deacetylase activity in Saos-2 cells. The administration of 4HR decreased histone deacetylase activity and the expression level of HDAC1, 3, 4, and 5. (A) Histone deacetylase activity decreased upon 4HR administration. When compared to untreated control, 10 and 100 μM 4HR group showed significantly lower values at 8 and 24 h after administration (* p < 0.05). (B) The level of acetylated histone 3 (Ac-H3) increased by 4HR administration. When Ac-H3 to H3 ratio of untreated control was compared, 4HR administered groups showed significantly higher values (* p < 0.05). (C) Expression level of HDAC1, 3, 4, and 5 decreased by 4HR administration. When compared to untreated control, 10 and 100 μM 4HR group showed significantly lower values at 8 and 24 h after administration (* p < 0.05). (D) Protein acetylation level generally increased by 4HR administration, as shown in whole cellular lysates.
The expression level of HDAC1, 3, 4, and 5 was decreased by 4HR administration (Figure 1B). When the relative expression level of each HDAC to β-actin was compared among groups, the expression level in Saos-2 cells treated with 4HR (1–100 μM) significantly decreased (p < 0.001). In the post hoc test, 100 μM 4HR treatments resulted in a significantly lower expression level of HDAC1 compared with that in the untreated control group at 8 h (p = 0.038). When observed at 24 h after 4HR treatments, 10 and 100 μM 4HR treatments resulted in the significantly lower expression level of HDAC1 compared with the untreated control group (p = 0.006 and <0.001, respectively); 10 and 100 μM 4HR treatments resulted in the significantly lower expression level of HDAC3 compared with the untreated control group at 8 h (p < 0.001 and =0.018, respectively). When observed at 24 h after 4HR treatments, 10 and 100 μM 4HR treatments resulted in a significantly lower expression level of HDAC3 compared with in the untreated control group (p < 0.001); 10 and 100 μM 4HR treatments resulted in a significantly lower expression level of HDAC4 compared with in the untreated control group at 24 h (p = 0.007 and <0.001, respectively); 10 and 100 μM 4HR treatments resulted in significantly lower expression level of HDAC5 compared with in the untreated control group at 24 h (p = 0.001 and <0.001, respectively).
The acetylated histone 3 (Ac-H3) was increased by 4HR administration (1–100 μM;
Figure 1C). When the relative expression ratio of Ac-H3 to H3 was compared among groups, the ratio in Saos-2 cells treated with 4HR (1–100 μM) increased significantly (
p < 0.001). In the post hoc test, 4HR treatments (1–100 μM) resulted in a significantly higher ratio of AC-H3 to H3 compared with that in the untreated control group (
p = 0.003 for 1 μM at 2 h and
p < 0.001 for the other groups). The protein acetylation level for whole-cell lysates demonstrated that 4HR administration increased protein acetylation level (
Figure 1D). Mitochondria are the main cellular power factories for the production of ATP—a cellular energy source. Oxygen consumption was decreased by 4HR administration (
Supplementary Figure S1). Thereafter, the production of ATP was decreased by 4HR administration in Saos-2 cells (
Supplementary Figure S2). Mitochondrial membrane potential (MMP) was increased by 20% after 100 μM 4HR administration (
Supplementary Figure S3). The administration of 4HR decreased cytochrome c levels at 100 μM, but not other concentrations in the mitochondrial fraction (
Supplementary Figure S4). Interestingly, 1 and 10 μM 4HR administration slightly increased the cytochrome c level of mitochondria without statistical significance (
p > 0.05). Decreased cytochrome c at 100 μM of 4HR was not detected in the cytoplasmic fraction.
3. 4HR Administration Increases Dental Hard Tissue Formation and Incisor Eruption Rate in Rats
Administration of 4HR increased the expression level of TGF-β1, OSX, DSPP, PTHrP-R, BMP-2/4, and Runx2, in dose- and time-dependent manner (Fig. 2).
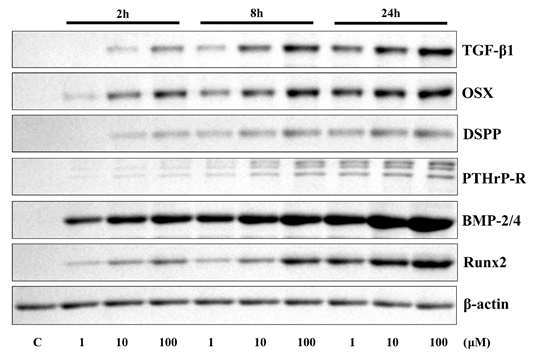
Figure 2. Results of western blotting assay for cell culture. Application of 4HR increased the expression level of TGF-β1, OSX, DSPP, PTHrP-R, BMP-2/4, and Runx2 in a time- and dose-dependent manner.
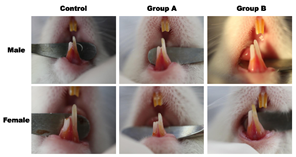
Figure 3. Results of tooth eruption study. Representative findings after 4HR administration. Administration of 4HR increased the rate of incisor eruption.
Administration of 4HR increased the rate of incisor eruption in both sexes of rats (Fig. 3). When the total amount of eruption was compared between males and females, there was no significant difference between the sexes in the same group (P > 0.05). The total amount of eruption in males at 15 days after 4HR administration was 21.75 ± 0.83 mm, 23.49 ± 0.94 mm, and 24.76 ± 0.91 mm in control, Group A, and Group B, respectively. Difference across the groups was statistically significant (P = 0.001). In post-hoc test, the difference between control and experimental groups was statistically significant (P = 0.028 and 0.001 for Groups A and B, respectively). However, there was no statistically significant difference between Groups A and B (P > 0.05).
The total amount of eruption in females after 15 days of 4HR administration was 22.00 ± 1.66 mm, 23.55 ± 1.93 mm, and 26.22 ± 1.85 mm in control, Group A, and Group B, respectively. Difference across the groups was statistically significant (P = 0.010). In post-hoc test, the difference between control and Group B was statistically significant (P = 0.010). However, there was no statistically significant difference between Groups A and B (P > 0.05).
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168935