Periodontitis (PD) is an infection-driven inflammatory disease of periodontal tissues caused by pathogenic microorganisms, which have been characterized by disruption of the tooth-supporting structures. In periodontal disease treatment, the conventional route of drug administration has many drawbacks such as poor biodistribution, low selectivity of the therapeutic effect, burst release of the drug, and damage to healthy cells. To overcome these difficulties, controlled drug delivery systems have been evolved as an alternative method to address oral infectious disease ailments. The use of drug delivery devices proves to be an excellent auxiliary method in enhancing the quality and effectiveness in periodontitis treatment, which includes inaccessible periodontal pockets. In the literature, there have been described as many various polymer-based delivery systems such as hydrogels, liposomes, micro-, and nanoparticles, which can be used in the treatment of chronic periodontal disease. The review shows the current state of knowledge regarding the applications of various polymer-based delivery systems such as hydrogels, liposomes, micro-, and nanoparticles in the treatment of chronic periodontal disease.
- Periodontitis (PD)
- Drug Delivery Systems (DDS)
- Polymeric Drug Delivery Systems
- Drug Release
- Periodontal Treatment
- Pathogenesis of Periodontitis Diseases
1. Definition
Periodontitis (PD) is an infection-driven inflammatory disease of periodontal tissues caused by pathogenic microorganisms, which have been characterized by disruption of the tooth-supporting structures.
2. Introduction
Periodontitis (PD) is a localized chronic inflammatory disease of periodontal tissues caused by pathogenic microorganisms and characterized by disruption of the tooth-supporting structures [1]. The inflammation is primarily localized to the gum but penetrates deeper if left untreated, creating pockets that host anaerobic bacteria which can then lead to further erosion of the tooth attachment and eventually to tooth loss. Periodontitis includes various degenerative and inflammatory states of the tissue surrounding the tooth, e.g., gingival, periodontal ligaments, cementum, and alveolar bone.
According to the World Health Organization report, it is one of the world’s most widespread chronic ailments occurring after the age of 35 [2][3]. Figure 1 shows a schematic depiction of periodontal disease.
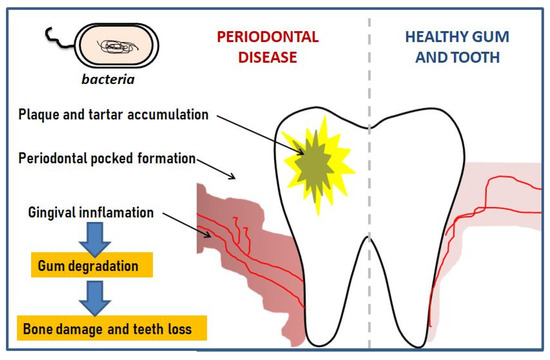
Figure 1. Schematic development of periodontal disease.
The current treatment of periodontal disease involves oral hygiene such as mechanical cleaning (brushing and flossing), together with the use of dentifrices and antibacterial mouthwashes. Non-surgical treatment methods for periodontitis include mechanical scaling and root planning [4]. Additionally, antibiotics, especially tetracyclines, β-lactam, and nitroimidazole antibiotics are mostly used for the treatment of periodontal disease [5].
Conventional drug administration routes are the most popular methods of drug treatment due to certain advantages, such as ease of use and a very high degree of dosage flexibility. This method is used to eliminate or inhibit gingival bacteria flora, decrease inflammation, and help to discontinue bone desorption. However, conventional drug administration pathways also have many drawbacks such as serious side effects, poor biodistribution, low selectivity of the therapeutic effect, burst release of the drug, and damage to healthy cells [6],[7]. The efficacy of conventional antimicrobial therapy has often been hampered by the difficulties of achieving and maintaining an appropriate concentration of curative agents at the periodontal site when a low dose of the drug is given. To overcome these limitations, a more effective approach to administering drugs into the periodontal pocket needs to be developed.
Recently, there has been growing interest in controlled drug delivery systems as a whole and as a potential method to address oral infectious disease ailments in particular [8]. Drug delivery systems (DDS) are used vehicles to transport therapeutic agents including antibodies, peptides, vaccines, drugs, and enzymes to a target location and safely achieve the desired therapeutic effect. Thus, the administration and efficacy of these pharmaceutical compounds are improved [9],[10]. The effectiveness of medicine has increased with the development of the fabrication of drug delivery carriers, which can administer accurate doses of drugs, minimize side-effects, and improve healthcare treatment of patients [5][11].
Polymers continue to play an important role in the development of drug delivery technology due to their favorable physicochemical properties and offering the possibility of further modifications. Advanced polymer delivery systems have been developed, in recent years thanks to the innovations in chemical engineering technology. Modern advances in drug delivery systems are now based on the design of polymers adapted to specific drugs and designed to perform distinct biological functions. To control the release of drugs and other active substances, a wide range of natural and synthetic origin polymer-carriers have been used. Polymers are potential drug carrier candidates but they must present some characteristics such as efficacy, hydrophilicity, lack of immunogenicity, lack of biological activity, and the presence of functional groups for covalent coupling of drugs or target moieties [12][13][14].
In recent years the field of controlled release drug delivery have been utilizing biodegradable polymers that can biodegrade within the body, such as polylactides (PLA), polyglycolide (PGA), poly(lactide-co-glycolides) (PLGA), polyanhydrides, polyorthoesters, all of which were previously known for other medical applications. The greatest advantage of these biodegradable polymers is that they are broken down into biologically tolerable molecules that are metabolized and removed from the body via normal metabolic pathways [15]. Apart from all these advantages, the use of polymeric carriers also has some limitations. For instance, naturally occurring polymers although highly abundant and biodegradable are difficult to reproduce and purify whereas, empty non-biodegradable polymers delivery devices needed to be removed by surgery after releasing the drug at the targeted site. Moreover, biodegradable materials do produce degradation byproducts that must be tolerated within the biological environment and therefore, both the desired and unwanted by-products must be carefully tested.
Polymer therapeutics include linear or branched polymer chains which act either as the bioactive molecule, e.g., polymeric drug or as the inert carrier to which a drug can be covalently linked, e.g., polymer-drug conjugates, dendrimers, polymeric micelles, nanocapsules, nanospheres, hydrogels, liposomes, etc. However, in order to successfully be used in controlled drug delivery formulations, a polymeric carrier must be chemically inert and free of leachable impurities. In other words, it must have an appropriate physical structure, with minimal undesired aging, and be readily processable [16].
The elaborated periodontal pocket drug delivery devices based on polymers improve the antimicrobial efficacy and demonstrate many consequent clinical benefits. It is worth mentioning that local sustained administration of drugs into the periodontal pockets in the mouth is challenging due to the continuous secretion of saliva. This facilitates for the implant to leave the periodontal pocket easily and it could then be swallowed by the patient [17]. Usage of a local drug delivery system in the treatment of periodontitis allows to achieve and maintain a greater concentration of drug at the diseased area than possible with conventional therapy, preventing the side effects of free-drugs.
3. The Pathogenesis of Periodontitis Diseases
In healthy individuals, the periodontium is mostly colonized by harmless, commensal bacterial communities [18]. A proper balance in their composition prevents the immune system from a potentially detrimental reaction. A dental plaque is defined as a polymicrobial biofilm on the surface of the tooth and tooth root. Presumably, due to poor hygiene or risk factors, excessive dental plaque may accumulate leading to the destruction of periodontal connective tissue and alveolar bone, the disease known as periodontitis [19]. One more important process is connected with this pathological state: a shift in microbial composition from mostly Gram-positive to mostly Gram-negative bacterial species [20][21]. Nevertheless, the mechanism causing the bacterial transition from one state to the other remains to be elucidated and a model implicating Gram-negative bacteria as the sole etiologic factor is rather oversimplistic [21].
The gingival epithelium constitutes an interface between the human organism and complex microbial community inhabiting the oral cavity. Hence, it is constantly challenged by numerous bacterial species. Any perturbations must be immediately faced by the immune system. The adaptive immune response requires several days before it may start to combat microbes, therefore innate immunity plays a critical role in the early stages of infection [22]. Pathogen associated molecular patterns (PAMPs) are defined as conserved microbial moieties that trigger the innate arm of the immune system which, in turn, directs adaptive immunity. Usually, they are crucial for microbial survival, thus their evolution rate is strongly inhibited. As PAMPs may serve diverse pathogen structures like lipopolysaccharide (LPS), flagellin, or fimbriae [23][24][25][26]. Pattern recognition receptors (PRRs), located on/in immunologically-relevant cells, are host receptors involved in PAMP recognition and initiation of the inflammatory response. Usually, such a response is beneficial, because it restricts infection and allows for pathogen removal from the host organism, but when the reaction is exaggerated or lasts too long, the inflammation may lead to detrimental effects on the host tissues, including tissue injury, perfusion defects, chronic inflammation state, and sepsis [27].
A healthy junctional epithelium is rich in many innate mediators [19], and an appropriate expression of interleukin 8 (IL-8), intracellular adhesion molecules (ICAMs) and E-selectin enables neutrophils’ presence within the tissue. Their role is to keep bacteria forming dental plaque under control [28][29]. Since periodontitis is related to a shift from Gram-positive to predominately Gram-negative flora, one of the essential steps for host defense is to correctly detect such a transition. The best characterized PAMP of Gram-negative bacteria is LPS, a major component of the outer membrane. When a pathogen invades the tissues, LPS might be released from the cell surface. Subsequently, it binds to LPS binding protein (LBP), complexes with a soluble or membrane-anchored CD14, and as the LPS-LBP-CD14 complex is transferred to another complex, namely TLR4-MD-2 (Figure 2) [30].
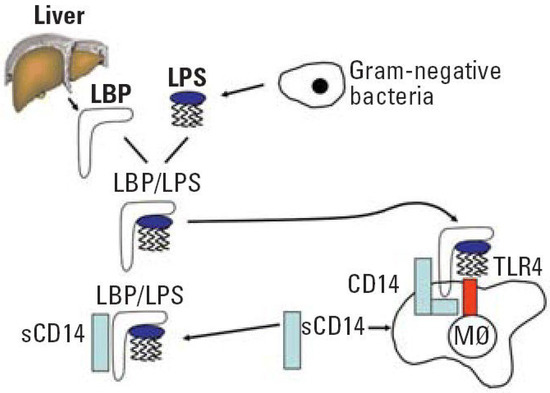
Figure 2. LPS recognition system. Abbreviation: LPS, lipopolysaccharide; LBP, lipopolysaccharide binding protein; CD, cluster of differentiation; sCD14, soluble CD14; TLR4, toll-like receptor 4; M∅ macrophage. Reprinted with permission from [30]. Copyright Environ. Health Perspect., 2006.
After activation, TLR-4 undergoes oligomerization and triggers downstream molecular cascade which ends with the synthesis of the proinflammatory cytokines and type-1 interferon. The effect of this pathway is then intensification of the immune response and, under standard conditions, infection eradication [25]. An interesting finding made by Ren and colleagues [31] is that LBP levels in healthy gingival tissues were significantly higher when compared to tissue with already developed periodontitis. Nonetheless, whether this is a cause or effect of the growing infection is not established. It is worth noting that other PRRs like TLR2 may induce a destructive inflammatory response [32].
One of the major symptoms of periodontitis is bone loss. Once the immune system fails to limit a local gingival infection, the inflammatory state expands finally reaching alveolar bone and causing severe tissue destruction [33]. In healthy individuals, bone remodeling understood as bone resorption and bone formation is equable [34]. The receptor–activator of nuclear factor-κB ligand (RANKL) /osteoprotegerin (OPG) ratio maintains this equilibrium. OPG functions as a soluble receptor for RANKL, the ligand for the receptor–activator of nuclear factor-κB (RANK) receptor, which is produced by osteoblasts and exists either as a soluble or membrane-anchored protein The interaction between OPG and RANKL prevents RANKL from binding RANK, the receptor RANK present on the osteoclasts and their precursors. RANK binds RANKL and directs precursor cells to differentiate into ones producing enzymes breaking down a bone (Figure 3) [35]. Proinflammatory cytokines such as IL-1β, -6, -11, and -17 and TNF-α lead to greater RANKL expression and drop in the OPG levels [36]. It was proved that in periodontitis the RANKL/OPG balance is disturbed in favor of higher RANKL/OPG ratios [37][38].
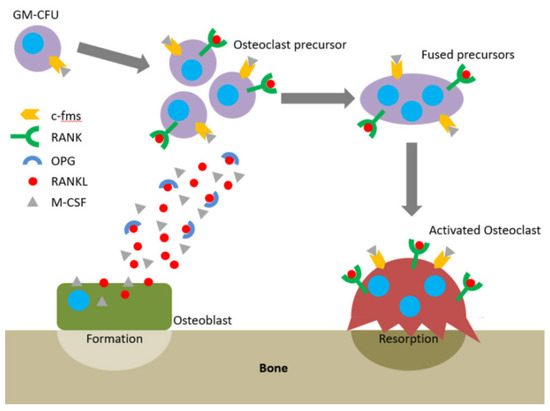
Figure 3. The RANKL/RANK/OPG axis and M-CSF direct osteoclastogenesis and activation. Reprinted with permission from [35]. Copyright Owen and Reilly (2018).
However, the bone loss process in periodontitis is more intricate than simple shifts in the RANKL/OPG ratio and involves more signaling molecules like IL-1, IL-6, TNF, or metalloproteinases [39][40][41]. The latter ones are a very important group from the periodontitis pathogenesis point of view. They are protein-degrading enzymes playing a variety of roles, including proteolysis of chemokines, receptors, inhibitors, and collagen, hence significantly influencing inflammation regulatory processes.
Collagen is a basic extracellular matrix component of connective tissue and its destruction results in remarkable tissue impairment [41] In periodontitis-affected tissues elevated levels of MMP-8 were reported [42] and increased activity of MMP-9 and MMP-13 were observed [43]. It seems that MMP-13 is particularly implicated in the periodontal soft tissue and alveolar bone destruction. MMPs and their detailed role in periodontal tissue breakdown are covered by numerous articles and reviews [41][43].
Although the current model of periodontitis attributes the most severe consequences like tissue defects and teeth loss to be the effects of the oral homeostasis disturbance and overly active immune response [19][41] rather than direct action of periopathogens, some interesting findings have been made in establishing its bacterial aetiology. Two bacterial complexes so-called “orange” and “red” are comprised of species believed to be the major aetiological factors of periodontitis. The orange complex appears first and includes anaerobic or microaerophilic Gram-negative species mainly from Prevotella, Fusobacterium, and Campylobacter genera. As the pathological state progresses, red-complex bacteria—Tannerella denticola, Tannerella forsythia, and Porphyromonas gingivalis start to multiplicate and colonize gingival tissue [44]. Among them, P. gingivalis is the best studied. It uses numerous strategies to circumvent the immune response that allows for a further thriving of surrounding bacterial community ultimately leading to chronic inflammation of periodontal tissues [45]. Examples of its virulence factors are lipid A 1- and 4′-phosphatases that alter LPS into a non-immunogenic form [46], fimbriae promoting colonization of epithelial cells and inducing CXCR4/TLR2 cross-talk which leads to immune response evasion [47] or Hgp44 [48] functioning as bacterial adhesion, to name a few. Although many sides of P. gingivalis biology has been unveiled, a lot of aspects remain to be investigated, and it is only the tip of the iceberg. Periodontitis affected tissues are inhabited by plenty of interrelated bacterial species that interact with the host immune mechanisms thus creating extremely complicated signaling and dependencies network.
Altogether, periodontitis begins with oral homeostasis changes, proceeds with bacterial communities’ expansion to a chronic inflammatory state. Finally, prolonged inflammation severely damages the gingival and alveolar bone tissue integrity.
4. Conclusions and Future Perspectives
Conventional treatment of periodontal disease is long-term, and the currently available therapeutic substances are insufficiently effective and highly toxic to humans. Likewise, there are frequent side effects of drug use like poor biodistribution, low selectivity of the therapeutic effect, burst release of the drug, and damage to healthy cells. The above side effects are generally caused by incorrect drug dosing. From the medical point of view, an interesting approach is the use of controlled drug release systems, in which dynamic development has been observed in recent years in treating periodontal disease. The solution is modern drug formulations that allow increased bioavailability (solubility, stability in vivo, membrane permeability), and improve the overall therapeutic effectiveness Polymeric drug carriers compared with other carriers have shown attractive properties such as biodegradability, tissue biocompatibility, and effectiveness for encapsulation of different antibiotics. In the literature, a large number of scientific articles on the formation of controlled release systems of antibacterial substances with the use of synthetic and natural polymers such as poly(d,l-lactide-co-glycolide), polylactide or chitosan has been observed. Various types of DDS have been developed as potential drug vehicles such as nanoparticles, hydrogels, liposomes, etc., which showed effective antibacterial action against various bacterial strains. Local drug delivery systems have been developed to provide controlled and sustained release of antibacterial agents in the vicinity environment of the inflammation site.
With the continuous development in the pharmaceutical fields, there is no doubt that drug delivery systems based on biopolymers can vastly improve the treatment of various infections and diseases. Targeted pharmacological therapy for periodontitis is an active area of ongoing research that needs to be further developed to evolve better therapeutic strategies and improve the patient experience. There is still a high demand for new, effective drug delivery systems for the treatment of periodontal disease, and further studies on this area are therefore highly anticipated.
The present review has demonstrated many different polymer-based drug delivery systems that can be potentially used to fight against periodontitis diseases. However, there are some challenges in using polymers as carriers in DDS for the treatment of periodontal disease, which should be resolved in the future. The first of them is related to a portfolio of appropriate polymers for application in DDS for periodontal treatment. To meet these requirements of effective drug delivery systems, the tailor-made polymers with relatively complex structures are increasingly used, which is associated with the complexity of their synthesis. That is why it is important to develop procedures that allow the synthesis of such polymeric carriers with high homogeneity (low dispersity) in a repeatable manner. The second direction, which can improve the comfort of patients can be the optimization of the size of devices used for local treatment. In this case, a promising effect can give the development of nanoscale intrapocket devices which through delivery even of a low dose of the drug, enable efficient treatment. This idea, however, requires the use of both suitable polymer materials, a method of processing them as well as the method of the preparation of the respective medical devices.
Currently, different antibiotics such as tetracyclines including doxycycline and minocycline, metronidazole, and chlorhexidine are used for local delivery, however, these are known to cause undesirable side effects, such as stomatitis, onycholysis, skin discoloration and decreased blood platelets, just to name a few. Therefore, it is important to implement a new trend utilizing natural products (i.e., drugs possessing antibiotic properties) in such systems. It is also expected that cooperation among polymer chemists, biologists, and dental specialists in the forthcoming future as well as results of their studies significantly broaden the knowledge of the possibility of using polymeric carriers of herbal active substances in the field of medical science, particularly in the field of periodontal disease treatment.
This entry is adapted from the peer-reviewed paper 10.3390/polym12071574
References
- Gayasuddin Khan; Ravi R. Patel; Sarita K. Yadav; Nagendra Kumar; Sundeep Chaurasia; Gufran Ajmal; Brahmeshwar Mishra; Brahmeshwar Mishra; Development, optimization and evaluation of tinidazole functionalized electrospun poly(ε-caprolactone) nanofiber membranes for the treatment of periodontitis. RSC Advances 2016, 6, 100214-100229, 10.1039/c6ra22072j.
- Poul Erik Petersen; Hiroshi Ogawa; Strengthening the Prevention of Periodontal Disease: The WHO Approach. Journal of Periodontology 2005, 76, 2187-2193, 10.1902/jop.2005.76.12.2187.
- Rajeshwari H R; Dinesh Dhamecha; Satveer Jagwani; Meghana Rao; Kiran Jadhav; Shabana Shaikh; Lakshmi Puzhankara; Sunil Jalalpure; H.R. Rajeshwari; Local drug delivery systems in the management of periodontitis: A scientific review.. Journal of Controlled Release 2019, 307, 393-409, 10.1016/j.jconrel.2019.06.038.
- Sim Yee Lim; Mali Dafydd; Jieji Ong; Launa A. Ord-McDermott; Emma Board-Davies; Kirsty Sands; David Williams; Alastair J. Sloan; Charles M. Heard; Mucoadhesive thin films for the simultaneous delivery of microbicide and anti-inflammatory drugs in the treatment of periodontal diseases. International Journal of Pharmaceutics 2020, 573, 118860, 10.1016/j.ijpharm.2019.118860.
- Deeksha Joshi; Tarun Garg; Amit K. Goyal; Goutam Rath; Advanced drug delivery approaches against periodontitis. Drug Delivery 2014, 23, 363-377, 10.3109/10717544.2014.935531.
- Zamani, F.; Jahanmard, F.; Ghasemkhah, F.; Amjad-Iranagh, S.; Bagherzadeh, R.; Amani-Tehran, M.; Latifi, M. Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–270.
- Nilu Jain; Gaurav K. Jain; Shamama Javed; Zeenat Iqbal; Sushama Talegaonkar; Farhan J. Ahmad; Roop K. Khar; Recent approaches for the treatment of periodontitis. Drug Discovery Today 2008, 13, 932-943, 10.1016/j.drudis.2008.07.010.
- Rania Hamed; Ala’A Aburezeq; Ola Tarawneh; Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Development and Industrial Pharmacy 2018, 44, 1488-1497, 10.1080/03639045.2018.1464021.
- Gary Greenstein; Alan Polson; The Role of Local Drug Delivery in the Management of Periodontal Diseases: A Comprehensive Review. Journal of Periodontology 1998, 69, 507-520, 10.1902/jop.1998.69.5.507.
- Aaron C. Anselmo; Samir Mitragotri; An overview of clinical and commercial impact of drug delivery systems. Journal of Controlled Release 2014, 190, 15-28, 10.1016/j.jconrel.2014.03.053.
- Sarah A. Stewart; Juan Domínguez-Robles; Ryan F. Donnelly; Eneko Larrañeta; Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications.. Polymers 2018, 10, 1379, 10.3390/polym10121379.
- Bo Wang; Shuo Wang; Qi Zhang; Yixuan Deng; Xiang Li; Liangyu Peng; Xianghao Zuo; Meihua Piao; Xin Kuang; Shihou Sheng; et al. Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomaterialia 2019, 96, 55-67, 10.1016/j.actbio.2019.05.044.
- F. De La Portilla; S. Pereira; M. Molero; F. De Marco; V. Perez-Puyana; A. Guerrero; A. Romero A; Alberto Romero; Microstructural, mechanical, and histological evaluation of modified alginate-based scaffolds. Journal of Biomedical Materials Research Part A 2016, 104, 3107-3114, 10.1002/jbm.a.35857.
- Ndidi C. Ngwuluka; Nelson A. Ochekpe; Oi Aruoma; Naturapolyceutics: The Science of Utilizing Natural Polymers for Drug Delivery. Polymers 2014, 6, 1312-1332, 10.3390/polym6051312.
- Garg, V.; Chawla, K.; Pawar, S.K; Nanotechnology controlled local drug delivery system for the treatment of periodontitisc. J. Adv. Med. Med. Res. 2018, 26, 1–17, https://doi.org/10.9734/JAMMR/2018/40828.
- Prashansa Agrawal.; Significance of Polymers in Drug Delivery System. Journal of Pharmacovigilance 2015, 3, e127, 10.4172/2329-6887.1000e127.
- Zhanhai Dong; Yining Sun; Yuhui Chen; Yan Liu; Chuhua Tang; Xiaozhong Qu; Injectable Adhesive Hydrogel through a Microcapsule Cross-Link for Periodontitis Treatment. ACS Applied Bio Materials 2019, 2, 5985-5994, 10.1021/acsabm.9b00912.
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E.; Bacterial diversity in human subgingival plaque.. J. Bacteriol. 2001, 183, 3770–3783, .
- Richard P. Darveau; Periodontitis: a polymicrobial disruption of host homeostasis. Nature Reviews Microbiology 2010, 8, 481-490, 10.1038/nrmicro2337.
- Purnima S. Kumar; Ann L. Griffen; Melvin L. Moeschberger; Eugene J. Leys; Identification of Candidate Periodontal Pathogens and Beneficial Species by Quantitative 16S Clonal Analysis. Journal of Clinical Microbiology 2005, 43, 3944-3955, 10.1128/jcm.43.8.3944-3955.2005.
- Purnima S. Kumar; Eugene J. Leys; Jennifer M. Bryk; Francisco J. Martinez; Melvin L. Moeschberger; Ann L. Griffen; Changes in Periodontal Health Status Are Associated with Bacterial Community Shifts as Assessed by Quantitative 16S Cloning and Sequencing. Journal of Clinical Microbiology 2006, 44, 3665-3673, 10.1128/jcm.00317-06.
- Lu, Q.; Jin, L.; Darveau, R.P.; Samaranayake, L.P.; Expression of human β-defensins-1 and -2 peptides in unresolved chronic periodontitis. J. Periodontal Res. 2004, 39, 221–227, .
- Hui Zeng; Huixia Wu; Valerie Sloane; Rheinallt M. Jones; Yimin Yu; Patricia Lin; Andrew T. Gewirtz; Andrew S. Neish; Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. American Journal of Physiology-Gastrointestinal and Liver Physiology 2006, 290, G96-G108, 10.1152/ajpgi.00273.2005.
- Mehmet A. Eskan; George Hajishengallis; Denis F. Kinane; Differential Activation of Human Gingival Epithelial Cells and Monocytes by Porphyromonas gingivalis Fimbriae. Infection and Immunity 2006, 75, 892-898, 10.1128/iai.01604-06.
- Yong-Chen Lu; Wen-Chen Yeh; Pamela S. Ohashi; LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145-151, 10.1016/j.cyto.2008.01.006.
- Je-Kyung Ryu; Soo Jin Kim; Sang-Hyun Rah; Ji In Kang; Hi Eun Jung; Dongsun Lee; Heung Kyu Lee; Jie-Oh Lee; Beom Seok Park; Tae-Young Yoon; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38-50, 10.1016/j.immuni.2016.11.007.
- Steven L. Raymond; David C. Holden; Juan C. Mira; Julie A. Stortz; Tyler J. Loftus; Alicia M. Mohr; Lyle L. Moldawer; Frederick A. Moore; Shawn D. Larson; Philip A. Efron; et al. Microbial recognition and danger signals in sepsis and trauma. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2017, 1863, 2564-2573, 10.1016/j.bbadis.2017.01.013.
- Karin Nylander; Bo Danielsen; Ole Fejerskov; Erik Dabelsteen; Expression of the Endothelial Leukocyte Adhesion Molecule-1 (ELAM-1) on Endothelial Cells in Experimental Gingivitis in Humans. Journal of Periodontology 1993, 64, 355-357, 10.1902/jop.1993.64.5.355.
- M. S. Tonetti; Martin A. Imboden; Niklaus P Lang; Neutrophil Migration Into the Gingival Sulcus Is Associated With Transepithelial Gradients of Interleukin-8 and ICAM-1. Journal of Periodontology 1998, 69, 1139-1147, 10.1902/jop.1998.69.10.1139.
- Darryl C. Zeldin; Peyton Eggleston; Martin D. Chapman; Giovanni Piedimonte; Harard Renz; David Peden; How Exposures to Biologics Influence the Induction and Incidence of Asthma. Environmental Health Perspectives 2006, 114, 620-626, 10.1289/ehp.8379.
- Lei Ren; Lijian Jin; Wai Keung Leung; Local expression of lipopolysaccharide-binding protein in human gingival tissues. Journal of Periodontal Research 2004, 39, 242-248, 10.1111/j.1600-0765.2004.00732.x.
- Elia Burns; Gilad Bachrach; Lior Shapira; Gabriel Nussbaum; Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption.. The Journal of Immunology 2006, 177, 8296-8300, 10.4049/jimmunol.177.12.8296.
- Dana T. Graves; D. Cochran; The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. Journal of Periodontology 2003, 74, 391-401, 10.1902/jop.2003.74.3.391.
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L; Osteoclast differentiation and activation. Boyle Ocl. Rev. 2003, 423, 337–342, .
- Robert Owen; Gwendolen C. Reilly; In vitro Models of Bone Remodelling and Associated Disorders. Frontiers in Bioengineering and Biotechnology 2018, 6, 134, 10.3389/fbioe.2018.00134.
- Cochran, D.L.; Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576, 10.3389/fbioe.2018.00134.
- Tania Crotti; Malcolm D. Smith; Robert Hirsch; Steven Soukoulis; Helen Weedon; Maria Capone; Michael J. Ahern; D.R. Haynes; Receptor activator NF κB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. Journal of Periodontal Research 2003, 38, 380-387, 10.1034/j.1600-0765.2003.00615.x.
- Nawarat Wara-Aswapati; Rudee Surarit; Anek Chayasadom; Jason A. Boch; W Pitiphat; RANKL Upregulation Associated With Periodontitis andPorphyromonas gingivalis. Journal of Periodontology 2007, 78, 1062-1069, 10.1902/jop.2007.060398.
- R Assuma; T Oates; D Cochran; S Amar; Dana T. Graves; IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis.. The Journal of Immunology 1998, 160, 403–409, .
- Pamela J. Baker; Mark Dixon; R. Todd Evans; Lisa Dufour; Ellis Johnson; Derry C. Roopenian; CD4+ T Cells and the Proinflammatory Cytokines Gamma Interferon and Interleukin-6 Contribute to Alveolar Bone Loss in Mice. Infection and Immunity 1999, 67, 2804-2809, .
- Cavalla Franco; Hernández-Ríos Patricia; Timo Sorsa; Cláudia Cristina Biguetti; Hernández Marcela; Matrix Metalloproteinases as Regulators of Periodontal Inflammation. International Journal of Molecular Sciences 2017, 18, 440, 10.3390/ijms18020440.
- Päivi Mäntylä; M. Stenman; D. Kinane; T. Salo; K. Suomalainen; S. Tikanoja; T. Sorsa; Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. Journal of Periodontal Research 2006, 41, 503-512, 10.1111/j.1600-0765.2006.00897.x.
- Hernández Ríos, M.; Sorsa, T.; Obregón, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J.; et al. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017, .
- Rinkee Mohanty; Swati Joshi Asopa; M. Derick Joseph; Bhupender Singh; Jagadish Prasad Rajguru; K. Saidath; Uma Sharma; Red complex: Polymicrobial conglomerate in oral flora: A review.. Journal of Family Medicine and Primary Care 2019, 8, 3480-3486, 10.4103/jfmpc.jfmpc_759_19.
- Camille Zenobia; George Hajishengallis; Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 2015, 6, 236-243, 10.1080/21505594.2014.999567.
- Coats, S.R.; Jones, J.W.; Do, C.T.; Braham, P.H.; Bainbridge, B.W.; To, T.T.; Goodlett, D.R.; Ernst, R.K.; Darveau, R.P.; Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1-and 4’-phosphatase activities. Cell. Microbiol. 2009, 11, 1587–1599, .
- George Hajishengallis; Min Wang; Shuang Liang; Martha Triantafilou; Kathy Triantafilou; Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proceedings of the National Academy of Sciences 2008, 105, 13532-13537, 10.1073/pnas.0803852105.
- Mariko Naito; Eiko Sakai; Yixin Shi; Hiroshi Ideguchi; Mikio Shoji; Naoya Ohara; Kenji Yamamoto; Koji Nakayama; Porphyromonas gingivalis-induced platelet aggregation in plasma depends on Hgp44 adhesin but not Rgp proteinase. Molecular Microbiology 2006, 59, 152-167, 10.1111/j.1365-2958.2005.04942.x.
