Rheumatoid arthritis (RA) is a common chronic inflammation-mediated disorder having systematic complications. RA triggers a self-directed inflammatory and immunological cascade that culminates in joint destruction. Though a range of treatment options are available, none of them are without adverse effects, leading researchers to search for alternative solutions. Nanomedicine has emerged as a powerful therapeutic alternative, and selenium (Se) is an essential micronutrient trace element that has a crucial role in human health and disease. The potential of SeNPs can be attributed to the effect of functional groups bound to them, concentration, and most importantly to their nano range size. The antirheumatic effect of SeNPs is considerable due to its potential in amelioration of oxidative stress-mediated inflammation via downregulation of radical and nonradical species, markers of inflammation, and upregulation of inherent antioxidant defenses.
- selenium
- nanoparticles
- biological methods
- inflammation
- oxidative stress
- rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a common chronic inflammation-mediated disorder [1]. It is a long-lasting condition described as the inflammation of diarthrodial joints leading to symmetrical polyarthritis and synovial hyperplasia (swelling) that results in progressive destruction of cartilage and bones and loss of articular function that leads to the eventual deformation of joints [2]. Moreover, it is a systematic autoimmune disorder that can alter multiple organ systems [3].
The exact etiological milieu for RA is still not certain, but as an example of a chronic inflammation-mediated autoimmune disorder, it has been correlated to oxidative stress (OS), a state wherein a pool of reactive oxidative species (ROS) upregulates actively, either due to their enhanced generation, the decline in antioxidative defense mechanisms, or the combined effects of both, thus leading to altered redox signaling that is involved in the maintenance and progression of the disorder [4][5]. The therapeutic options for patients suffering from RA include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GC), and disease-modifying antirheumatic drugs (DMARDs), but all of these available therapeutic remedies have associated adverse effects [1]. Thus, there is a prominent need to develop and test novel drugs that intend to ameliorate inflamed synovial joints and mitigate bone damage. Selenium (Se) is an essential micronutrient trace element having a crucial role in normal human functioning and has prominent relevance to several pathophysiological conditions [2].
In this regard, one of the most promising therapeutic solutions for RA is ‘nanomedicine’ [3] and has captured quite the amount of attention. Selenium nanoparticles (SeNPs) have become the centerpiece of attention due to their exclusive physical and chemical properties [6]. The SeNPs play an essential role in the antioxidant defense system that is crucial for reduction of oxidative stress [7].
2. Serum Selenium Status in Rheumatoid Arthritis
In the past, lowered serum concentration of trace micronutrients has been demonstrated as a frequent event in autoimmune diseases [8]. Epidemiological reports proved that a low Se status can be a risk factor for RA, indicating the significance of antioxidants in controlling the maintenance and progression of the disease [9][10][11][12][13][14][15][16][17][18].
It has been reported that Se supplementation improves the condition of patients as well as reduces inflammation levels in experimental models, such as the granuloma pouch exudate, and in lupus mice or in adjuvant arthritis in rats [19]. Evidence has suggested that Se can decrease inflammation in autoimmune disorders [20].
3. Current RA Medication
Conventional treatment options for RA patients comprise NSAIDs, GC, conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), and biologic DMARDs (bDMARDs), and all these available therapeutic remedies have associated side effects. Table 1 summarizes the diverse treatment options available for RA and their associated side effects.
| Drugs | Mode of Mechanism | Side Effects |
|---|---|---|
| NSAIDs | Inhibition of COXs | Cardiovascular risk, gastro-intestinal disorders, and renal malfunction |
| GCs | Inhibition of phospholipid release | Cardiovascular disorders, osteoporosis, insulin resistance, skin thinning, hypertension, and obesity |
| Conventional synthetic DMARDs | Disease altering activities | Interstitial pneumonitis, myelosuppression, hepatic cirrhosis, retinopathies, hypersensitivity, and allergic reactions |
| Biologic DMARDs | Inhibitors to immune mediators | Bacterial infections and high costs |
| Targeted synthetic DMARDs | Intracellular blockers of tyrosine kinase | Infections, headaches, hypertension, nausea, diarrhea, and high cholesterol levels |
4. Nanomedicine as a Potential Solution
RA continues to be a challenging disorder because all the above-mentioned recommended therapies do not often lead to a cure and are linked to frequent drug resistance and related side effects [21]. Hence, it is crucial to develop and test novel drugs that target inflamed joints and mitigate damage. In this regard, one of the most promising therapeutic solutions for RA is nanomedicine [3]. Hundreds of diverse nanomedicine formulations have been prepared and assessed over the years for various kinds of maladies. However, about 50 of such formulations are at present approved for clinical usage, and several nanomedicines are going through trials [22]. Nanoparticles (NPs) are defined as nano-range submicroscopic particles that have unique properties such as large surface area, nano size, surface charge, and chemistry, solubility, and multifunctionality [23]. NPs are deemed as being in a transitional stage between individual molecules and the analogous bulk materials, which allows them to possess peculiar properties that are unique from their molecular and bulk analogue counterparts [23][24]. Based on their unique properties, nanoscale materials and devices can interact with biomolecules from both the inside and on the cell surface that has the potential to detect disorders and deliver treatments. Hence, NPs have revolutionized healthcare as they facilitate research and development, help with early detection, enhance molecular imaging, and enable prevention, diagnosis, and control [25].
5. Selenium Nanoparticles
SeNPs have received attention due to their exclusive physical and chemical properties (i.e., mechanical, electrical, catalytic, and opt-magnetic properties) that are exhibited when this element is scaled down to the nano range as a result of high spatial confinement of nanomaterials, high surface-to-volume ratio, and large surface energy [26][27][28]. SeNPs, due to remarkable photoreactive, biocidal, anticancer, antidiabetic, antioxidant, antimicrobial, and anti-inflammatory properties in the healthcare arena, is being used in antimicrobial coatings, diagnostics, medical devices, nutritional supplements, and nanotherapeutics [29][30]. SeNPs have significantly emerged as a dual-targeting modality with both pro-oxidant and antioxidant potential dependent on subsequent duration, dose, and frequency as well as oxidation state. The pro-oxidant potential of SeNPs has been exploited in anticancer agents (chemotherapeutic drugs carriers). These NPs fundamentally localize in the malignant cells and lead to the production of reactive oxygen species, and hence cause cytotoxicity. The pro-oxidant mechanism of SeNPs follows the reduction of nano selenium via thioredoxin- and glutaredoxin-mediated redox signaling that leads to the generation of Se2− anion through the consumption of NADPH+H+ and stimulated production of ROS [31]. SeNPs play an important role in the antioxidant defense system, which is essential for reducing oxidative stress [7]. Se is an integral part of selenoproteins, such as glutathione peroxidases (GPxs) and TrxRs, which are needed for several biochemical reactions involved in normal antioxidant defenses [32]. SeNPs have been studied in different inflammation and redox imbalance-mediated disorders, such as cancer, diabetes, nephritis, and arthritis, and showed potential remedial uses [29].
5.1. Pharmacokinetics and Toxicological Profile of SeNPs
Oral intake of NPs is regarded as the most suitable and cost-effective mode of supplementation. Nonetheless, the absorption of NPs is hindered by two gastrointestinal barriers: the intestinal mucosa and the mucus covering the intestinal mucosa [33]. In theoretical terms, NPs can pass through the intestinal epithelium via two transport methods: paracellular (between adjacent cells) or transcellular (through the cells) [34]. Intestinal epithelial cells can transfer NPs along with the mineral elements, though their ability is limited. Transcellular transport starts with endocytosis (pinocytosis or macropinocytosis) [35]. The absorption of the NPs depends on the size, surface hydrophobicity, and electric charge [36]. The epithelium of the digestive tract is comprised of lipids, resulting in a higher absorption rate of hydrophobic NPs than hydrophilic NPs. The absorption of 100 nm NPs in the digestive tract is about 15 to 250 times higher than that of larger NPs [37].
In a recent report, a comparison between SeNPs and selenomethionine (SeMet) in male C3H/HeJ mice to estimate the LD50 showed that SeNPs induce minor toxic effects compared to SeMet [38]. In short, SeNPs are less toxic, more bioavailable, and possess stronger biological properties than other organic and inorganic Se forms [39][40].
5.2. Protective Role of SeNPs against Rheumatoid Arthritis
Qamar et al. reported the potential of SeNPs prepared from Trachyspermum ammi against RA in BALB/c mice models. SeNPs exhibited correction in a manner independent of dose in the redox state through the upregulation of antioxidant defenses and a reduction of paw edema as compared to the diseased group [41]. Hence, the antiarthritic potential of SeNPs is perhaps due to the reduction of ROS, inflammation-related markers, and increase in antioxidant protection as established from recent research.
5.3. Role of SeNPs against ROS and Inflammation Markers
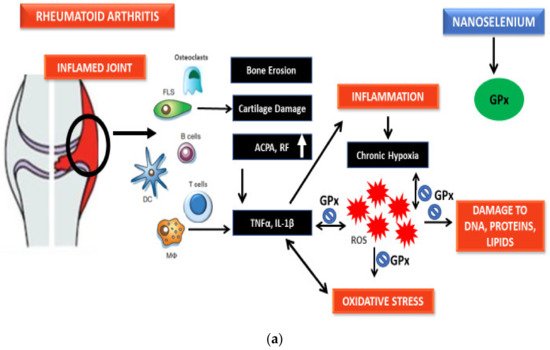
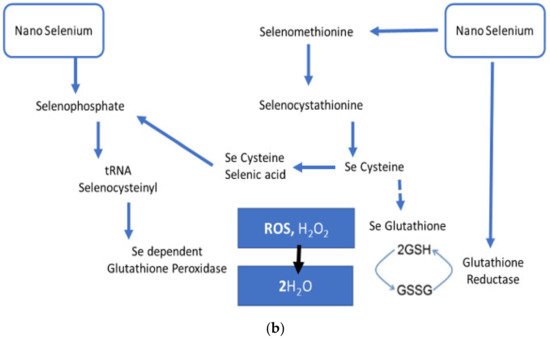
6. Significance of Biogenic Nanoparticles
6.1. Potential of Plant-Derived SeNPs
Zingiber officinale (ginger)-derived SeNPs have been tested against aluminum chloride-induced hepatorenal toxicity in rats and provided significant antioxidant benefits through reduction in GSH, SOD, GPx, and malondialdehyde (MDA) levels [58]. Ginger-extract-made SeNPs have also been reported to have antioxidant-mediated anti-inflammatory properties and improved nicotine-induced renal inflammation-mediated impairment in rats [59]. Menon et al. also reported the antioxidant potential of Zingiber officinale-derived SeNPs through DPPH tests [60]. Given that long-term treatment using DMARDs and NSAIDs leads to renal and hepatic toxic damage in rheumatoid patients, [61][62] ginger-derived SeNPs can be a possible option to diminish the treatment-associated toxic effects in rheumatoid patients. Kameswari et al. reported on the potent free radical scavenging and anti-inflammatory potential of SeNPs derived from Acalypha indica extract [63]. Other SeNPs derived from plants have also showed significant antioxidant potential [64][65][66][67].
6.2. Potential of SeNPs from Bacteria
6.3. Potential of SeNPs from Fungi
6.4. Potential of SeNPs from Proteins
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/nano11082005
References
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current therapeutic options in the treatment of rheumatoid arthritis. J. Clin. Med. 2019, 8, 938.
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777.
- Rubinstein, I.; Weinberg, G.L. Nanomedicines for chronic non-infectious arthritis: The clinician’s perspective. Nanomed. Nanotechnol. Biol. Med. 2012, 8, S77–S82.
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120.
- Oropeza-Moe, M.; Wisløff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2015, 31, 148–156.
- Boostani, A.; Sadeghi, A.A.; Mousavi, S.N.; Chamani, M.; Kashan, N. The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Sci. Vet. 2015, 43, 1–6.
- Iranifam, M.; Fathinia, M.; Rad, T.S.; Hanifehpour, Y.; Khataee, A.; Joo, S. A novel selenium nanoparticles-enhanced chemiluminescence system for determination of dinitrobutylphenol. Talanta 2013, 107, 263–269.
- Aaseth, J.; Munthe, E.; Førre, Ø.; Steinnes, E. Trace elements in serum and urine of patients with rheumatoid arthritis. Scand. J. Rheumatol. 1978, 7, 237–240.
- Hannonen, P.; Möttönen, T.; Oka, M. Serum selenium and rheumatoid arthritis. Scand. J. Rheumatol. 1985, 14, 440.
- Borglund, M.; Åkesson, A.; Åkesson, B. Distribution of selenium and glutathione peroxidase in plasma compared in healthy subjects and rheumatoid arthritis patients. Scand. J. Clin. Lab. Investig. 1988, 48, 27–32.
- Bacon, M.C.; White, P.H.; Raiten, D.J.; Craft, N.; Margolis, S.; Levander, O.A.; Taylor, M.L.; Lipnick, R.N.; Sami, S. Nutritional status and growth in juvenile rheumatoid arthritis. Semin. Arthritis Rheum. 1990, 20, 97–106.
- Jacobsson, L.; Lindgärde, F.; Manthorpe, R.; Akesson, B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 901–905.
- O’Dell, J.R.; Lemley-Gillespie, S.; Palmer, W.R.; Weaver, A.L.; Moore, G.F.; Klassen, L.W. Serum selenium concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 1991, 50, 376–378.
- Heliövaara, M.; Knekt, P.; Aho, K.; Aaran, R.; Alfthan, G.; Aromaa, A. Serum antioxidants and risk of rheumatoid arthritis. Ann. Rheum. Dis. 1994, 53, 51–53.
- Köse, K.; Doĝan, P.; Kardas, Y.; Saraymen, R. Plasma selenium levels in rheumatoid arthritis. Biol. Trace Elem. Res. 1996, 53, 51–56.
- Witkowska, A.M.; Kuryliszyn-Moskal, A.; Borawska, M.H.; Hukałowicz, K.; Markiewicz, R. A study on soluble intercellular adhesion molecule-1 and selenium in patients with rheumatoid arthritis complicated by vasculitis. Clin. Rheumatol. 2003, 22, 414–419.
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132.
- Pemberton, P.W.; Ahmad, Y.; Bodill, H.; Lokko, D.; Hider, S.L.; Yates, A.P.; Walker, M.G.; Laing, I.; Bruce, I.N. Biomarkers of oxidant stress, insulin sensitivity and endothelial activation in rheumatoid arthritis: A cross-sectional study of their association with accelerated atherosclerosis. BMC Res. Notes 2009, 2, 1–7.
- Parnham, M.J.; Winkelmann, J.; Leyck, S. Macrophage, lymphocyte and chronic inflammatory responses in selenium deficient rodents. Association with decreased glutathione peroxidase activity. Int. J. Immunopharmacol. 1983, 5, 455–461.
- Majeed, W.; Zafar, M.; Bhatti, A.; John, P. Therapeutic potential of selenium nanoparticles. J. Nanomed. Nanotechnol. 2018, 9, 1000487.
- Pham, C.T. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 607–619.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387.
- Jolly, J.; Rauf, M.A.; Ahmad, Z. Selenium nanoparticles: Small is the new big: Mini review. Open J. Chem. 2020, 6, 013–016.
- Taylor, R.; Walton, D.R. The chemistry of fullerenes. Nature 1993, 363, 685–693.
- Fymat, A. Recent developments in nanomedicine research. J. Nanomed. Res. 2016, 4, 00096.
- Cao, G. Chapter 1, Introduction. In Nanostructures and Nanomaterials: Synthesis, Properties and Applications; Cao, G., Ed.; Imperial College: London, UK, 2004; pp. 1–14.
- Yuwen, L.; Wang, L. Chapter 11.5, Nanoparticles and quantum dots. In Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; Devillanova, F., Mont, W.W.D., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2013; pp. 232–260.
- Appenzeller, T. The man who dared to think small. Science 1991, 254, 1300–1302.
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812.
- Vrček, I.V. Selenium nanoparticles: Biomedical applications. In Selenium; Springer: Berlin/Heidelberg, Germany, 2018; pp. 393–412.
- Ranjitha, V.; Rai, V.R. Selenium nanostructure: Progress towards green synthesis and functionalization for biomedicine. J. Pharm. Investig. 2021, 51, 117–135.
- Gladyshev, V.N.; Arnér, E.S.; Berry, M.J.; Brigelius-Flohé, R.; Bruford, E.A.; Burk, R.F.; Carlson, B.A.; Castellano, S.; Chavatte, L.; Conrad, M. Selenoprotein gene nomenclature. J. Biol. Chem. 2016, 291, 24036–24040.
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570.
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27.
- Buono, C.; Anzinger, J.J.; Amar, M.; Kruth, H.S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Investig. 2009, 119, 1373–1381.
- Plapied, L.; Duhem, N.; des Rieux, A.; Préat, V. Fate of polymeric nanocarriers for oral drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 228–237.
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210.
- Sonkusre, P. Specificity of Biogenic Selenium Nanoparticles for Prostate Cancer Therapy With Reduced Risk of Toxicity: An in vitro and in vivo Study. Front. Oncol. 2020, 9, 1541.
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268.
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31.
- Qamar, N.; John, P.; Bhatti, A. Toxicological and Anti-Rheumatic Potential of Trachyspermum ammi Derived Biogenic Selenium Nanoparticles in Arthritic Balb/c Mice. Int. J. Nanomed. 2020, 15, 3497–3509.
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383.
- Steinbrenner, H.; Alili, L.; Bilgic, E.; Sies, H.; Brenneisen, P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic. Biol. Med. 2006, 40, 1513–1523.
- Steinbrenner, H.; Steinbrenner, H.; Bilgic, E.; Steinbrenner, H.; Bilgic, E.; Alili, L.; Sies, H.; Brenneisen, P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic. Res. 2006, 40, 936–943.
- Jeong, D.-w.; Kim, T.S.; Chung, Y.W.; Lee, B.J.; Kim, I.Y. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002, 517, 225–228.
- Li, N.; Gao, Z.; Luo, D.; Tang, X.; Chen, D.; Hu, Y. Selenium level in the environment and the population of Zhoukoudian area, Beijing, China. Sci. Total Environ. 2007, 381, 105–111.
- Duntas, L. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447.
- Kahya, M.C.; Naziroğlu, M.; Çiğ, B. Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj. 2015, 29, 1490–1496.
- Vieira, A.T.; Silveira, K.D.; Arruda, M.C.; Fagundes, C.T.; Gonçalves, J.L.; Silva, T.A.; Neves, M.J.; Menezes, M.A.; Nicoli, J.R.; Teixeira, M.M. Treatment with Selemax®, a selenium-enriched yeast, ameliorates experimental arthritis in rats and mice. Br. J. Nutr. 2012, 108, 1829–1838.
- Van Cauwenbergh, R.; Robberecht, H.; Van Vlaslaer, V.; Deelstra, H. Comparison of the serum selenium content of healthy adults living in the Antwerp region (Belgium) with recent literature data. J. Trace Elem. Med. Biol. 2004, 18, 99–112.
- Bryant, R.W.; Bailey, J.M. Altered lipoxygenase metabolism and decreased glutathione peroxidase activity in platelets from selenium-deficient rats. Biochem. Biophys. Res. Commun. 1980, 92, 268–276.
- Al-Najjar, S.N.; Hussein, B. Salivary Selenium and Glutathione Peroxidase among Group of pregnant Women in Relation to Periodontal Condition. Int. J. Sci. Res. IJSR 2016, 6, 1238–1244.
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743.
- Pagmantidis, V.; Méplan, C.; van Schothorst, E.M.; Keijer, J.; Hesketh, J.E. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am. J. Clin. Nutr. 2008, 87, 181–189.
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Δ12, 14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007, 282, 17964–17973.
- Vunta, H.; Belda, B.J.; Arner, R.J.; Channa Reddy, C.; Vanden Heuvel, J.P.; Sandeep Prabhu, K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2008, 52, 1316–1323.
- Mizutani, T.; Goto, C.; Totsuka, T. Mammalian selenocysteine tRNA, its enzymes and selenophosphate. J. Health Sci. 2000, 46, 399–404.
- Al-Kahtani, M.; Morsy, K. Ameliorative effect of selenium nanoparticles against aluminum chloride-induced hepatorenal toxicity in rats. Environ. Sci. Pollut. Res. 2019, 26, 32189–32197.
- Zahran, W.E.; Elsonbaty, S.M.; Moawed, F.S. Selenium nanoparticles with low-level ionizing radiation exposure ameliorate nicotine-induced inflammatory impairment in rat kidney. Environ. Sci. Pollut. Res. 2017, 24, 19980–19989.
- Menon, S.; KS, S.D.; Agarwal, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci. Commun. 2019, 29, 1–8.
- Sotoudehmanesh, R.; Anvari, B.; Akhlaghi, M.; Shahraeeni, S.; Kolahdoozan, S. Methotrexate hepatotoxicity in patients with rheumatoid arthritis. Middle East J. Dig. Dis. 2010, 2, 104.
- Icardi, A.; Araghi, P.; Ciabattoni, M.; Romano, U.; Lazzarini, P.; Bianchi, G. Kidney involvement in rheumatoid arthritis. Reumatismo 2003, 55, 76–85.
- Kameswari, S.; Narayanan, A.L.; Rajeshkumar, S. Free radical scavenging and anti-inflammatory potential of Acalypha indica mediated selenium nanoparticles. Drug Invent. Today 2020, 13, 348–351.
- Vyas, J.; Rana, S. Antioxidant activity and biogenic synthesis of selenium nanoparticles using the leaf extract of aloe vera. Int. J. Curr. Pharm. Res. 2017, 9, 147–152.
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931.
- Kokila, K.; Elavarasan, N.; Sujatha, V. Diospyros montana leaf extract-mediated synthesis of selenium nanoparticles and their biological applications. New J. Chem. 2017, 41, 7481–7490.
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116.
- Xu, C.; Qiao, L.; Ma, L.; Yan, S.; Guo, Y.; Dou, X.; Zhang, B.; Roman, A. Biosynthesis of polysaccharides-capped selenium nanoparticles using Lactococcus lactis NZ9000 and their antioxidant and anti-inflammatory activities. Front. Microbiol. 2019, 10, 1632.
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585.
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491.
- Qiao, L.; Dou, X.; Yan, S.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020, 11, 3020–3031.
- Tachecí, I.; Bradna, P.; Douda, T.; Baštecká, D.; Kopáčová, M.; Rejchrt, S.; Lutonský, M.; Soukup, T.; Bureš, J. Small intestinal injury in NSAID users suffering from rheumatoid arthritis or osteoarthritis. Rheumatol. Int. 2016, 36, 1557–1561.
- Afzal, B.; Yasin, D.; Husain, S.; Zaki, A.; Srivastava, P.; Kumar, R.; Fatma, T. Screening of cyanobacterial strains for the selenium nanoparticles synthesis and their anti-oxidant activity. Biocatal. Agric. Biotechnol. 2019, 21, 101307.
- Wang, J.; Zhang, Y.; Yuan, Y.; Yue, T. Immunomodulatory of selenium nano-particles decorated by sulfated Ganoderma lucidum polysaccharides. Food Chem. Toxicol. 2014, 68, 183–189.
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 1–15.
- Attalla, M.G.; Singh, S.B.; Khalid, R.; Umair, M.; Epenge, E. Relationship between Ulcerative Colitis and Rheumatoid Arthritis: A Review. Cureus 2019, 11, e5695.
- Dobias, J.; Suvorova, E.I.; Bernier-Latmani, R. Role of proteins in controlling selenium nanoparticle size. Nanotechnology 2011, 22, 195605.
- Lenz, M.; Kolvenbach, B.; Gygax, B.; Moes, S.; Corvini, P.F. Shedding light on selenium biomineralization: Proteins associated with bionanominerals. Appl. Environ. Microbiol. 2011, 77, 4676–4680.
- Xu, D.; Yang, L.; Wang, Y.; Wang, G.; Rensing, C.; Zheng, S. Proteins enriched in charged amino acids control the formation and stabilization of selenium nanoparticles in Comamonas testosteroni S44. Sci. Rep. 2018, 8, 1–11.
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. Sodium selenite/selenium nanoparticles (SeNPs) protect cardiomyoblasts and zebrafish embryos against ethanol induced oxidative stress. J. Trace Elem. Med. Biol. 2015, 32, 135–144.
