Using several in vivo designs, antigen-presenting cells, including macrophages and dendritic cells (DCs), are identified in the pulpal tissue before tertiary dentin deposition under the afflicted area. However, the precise nature of this phenomenon and its relationship to inherent pulp cells are not yet clarified. This literature review aims to discuss the role of pulpal DCs and their relationship to progenitor/stem cells, odontoblasts or odontoblast-like cells, and other immunocompetent cells during physiological and pathological dentinogenesis.
- cell differentiation
- dental pulp
- dendritic cells
- dentin
- extracellular matrix proteins
- histocompatibility antigens class II
- macrophages
- odontoblasts
- stem cells
- tooth injuries
1. Introduction
The dental pulp is a specialized soft connective tissue of ectomesenchymal origin harboring distinct cell populations with specific functions involved in teeth production and maintenance [1][2][3]. Odontoblasts—dentin-forming cells—constitute the most specialized and important cells of the tissue [4][5][6], whereas fibroblasts—the most numerous cells of the pulp—are responsible for the synthesis and maintenance of the pulp extracellular matrix (ECM). Other important cell types include undifferentiated mesenchymal stem cells (MSCs)/progenitor cells, blood vessel components, nervous cells, and a wide variety of defense cells [1][2][3][7]. Under physiological conditions, the pulpal tissue executes self-regulatory mechanisms that maintain the equilibrium of these cell populations [8][9]. Previous studies in murine and human teeth demonstrated that the dental pulp has low proliferative activity, except for the apices of developing roots or the apical end of incisors in rodents [2][7][10][11]. Likewise, other studies have proven that apoptosis, or programmed cell death, occurs at low rates in the normal dental pulp of continuously growing incisor in rats and in the pulps of human mature teeth throughout life, regulating the turnover of odontoblasts, cells of the subodontoblastic layer, and fibroblasts [2][11][12][13][14][15]. Therefore, given the stable characteristics of the dental pulp under physiological conditions, it is essential to understand the tissue to the extent of its responsiveness after exogenous stimuli to the tooth.
Exogenous transdentinal stimuli, such as dental carious lesions, abrasion, attrition, dental traumatisms and restorative procedures, induce harmful variations in the odontoblast layer at the afflicted site, eliciting an immunocompetent response in the pulpal tissue [7][16][17][18][19]. Odontoblasts are considered as the first line of defense against pathogen invasion, facilitating the initiation, development, and maintenance of the immune/inflammatory response. The release of proinflammatory cytokines by the afflicted odontoblasts triggers specific intracellular signaling pathways involving nuclear factor-κB and mitogen-activated protein kinase p38 that promote the recruitment of leukocytes and antigen-presenting cells (APCs), including macrophages and immature dendritic cells (DCs) [4][5][6][20][21][22]. After the activation of the pulpal cellular network, a newly formed pathological dentin matrix is steadily deposited beneath the injury site. This calcified matrix, also known as tertiary dentin, can be further classified as either reactionary or reparative dentin based on the differences in the exogenous stimuli intensity to the tooth and the biological events in the pulpal tissue. Thus, the tertiary dentin matrix secreted by surviving odontoblasts in response to a mild stimulus is defined as reactionary dentin; whereas reparative dentin is defined as the matrix deposited beneath the afflicted area by newly differentiated odontoblast-like cells after the death of the original odontoblasts due to severe injuries [7][23][24][25][26][27][28][29][30][31][32][33]. The latter implies a more complex process involving important biological mechanisms, such as apoptosis, cell proliferation, and cell differentiation, which remain to be elucidated at cellular and gene levels [34][35][36][37][38]. APCs are also critical during the initial defense reaction. Previous in vivo studies have shown various patterns for these cells at the onset of the healing process. For instance, macrophages are located in the central portion of the pulp, whereas immature DCs can be detected in the odontoblast layer [7][16][39][40][41][42][43][44][45][46][47][48][49][50][51]. DCs are sentinels that maintain immune homeostasis, orchestrating the immune system’s components for a favorable effect in an organism [52][53]. Besides its main role in the host-response against pathogens, the paraodontoblastic location of these cells may suggest another important role during pulpal healing that needs to be clarified.
In the last decades, several animal models mimicking external injuries to the teeth have been established to analyze the behavior of distinct pulpal cell populations, with special emphasis on the crosstalk of dental pulp stem cells (DPSCs) and other inherent cells. Study designs using in vivo approaches included autogenic/allogenic tooth germ and whole-tooth replantation and transplantation [35][36][49][54][55][56][57][58][59], cavity preparations with or without pulp exposure [16][37][38][44][47][48][60][61], and root resection [62]. For instance, the injection of 5-bromo-2′-deoxyuridine in young animals at the optimal time demonstrated the localization of slow-cycling long-term label-retaining cells (LRCs). It was observed that LRCs in transplanted teeth maintain their proliferative and differentiation capacities despite extensive apoptosis occurring in the pulpal tissue of the transplant and play crucial roles in the pulpal healing process after exogenous stimuli, leading to the conclusion that dense LRCs are believed to be dental stem/progenitor cells in mature pulp tissue [63][64][65]. Therefore, animal models simulating different kinds of tooth injuries are necessary to identify the behavior of DPSCs in relation to other cellular lineages of the pulpal tissue, such as APCs [66][67][68]. Hence, this literature review discusses the possible role of dental pulp DCs and their relationship to odontoblasts, DPSCs, and other resident cells involved during physiological and pathological dentinogenesis.
2. Key Functions of DCs
DCs are professional APCs critical for the initiation and orchestration of the immune response [69]. DCs were described for the first time in 1973 by Dr. Ralph Steinman (Nobel Prize of Medicine and Physiology in 2011) as a heterogenous population of leukocytes originated from hematopoietic stem cells different from macrophages [52][70]. DCs are found in most parts of the human body, including the lymph nodes, skin, blood, spleen, lungs [71], and oral tissue [39][42][72], where they play a key role in maintaining immune homeostasis by both activating adaptive immunity and contributing to tolerance [53]. DCs can be classified into different subsets with specialized functions in immune responses to specific pathogens [73]. The two major DC subsets are plasmacytoid DCs (pDCs) and myeloid/conventional DCs (cDCs). These subsets are generally identified based on the lack of expression for T cells, B cells, natural killer (NK) cells, or other granulocyte-specific markers, high-level expression of major histocompatibility complex (MHC) class II, and lack of monocyte markers [69][74]. pDCs play an important role in the innate immune system because of their capacity to produce type I interferons upon viral infection and are also involved in tolerance or immune suppression in their immature state [75]. Differently, cDCs recognize bacterial components and produce proinflammatory cytokines to activate proinflammatory T-cell subsets and subsequently promote the recruitment of cytotoxic T lymphocytes. cDCs can be further divided into cDC1 and cDC2. Human cDC1s and cDC2s can be found in the blood and lymphoid and nonlymphoid tissue [75][76]. Thus, cDCs constitute a distinct lineage of cells with the capacity to seed tissue and maintain immune homeostasis in a steady state while rapidly responding to local insults and initiating and directing innate and adaptive immunity [76]. Immature cDCs accumulate at the inflammation site along with other phagocytic cells (e.g., macrophages) in response to the release of chemokines in this area. Antigens and their associated molecules will be later recognized and captured by cDCs through different mechanisms, including pinocytosis (macropinocytosis and micropinocytosis), phagocytosis, and receptor-medicated endocytosis. Subsequently, cDCs will migrate toward lymphoid tissue during their maturation process, and the antigenic peptides processed by cDCs will be presented on MHC class I and II molecules for interaction with different subsets of T cells [75][77][78]. Because pDCs are not the object of the analysis of this review, but cDCs (particularly those in the dental pulp), cDCs will simply be addressed as DCs.
3. Relationship between DCs and Odontoblasts or Newly Differentiated Odontoblast-like Cells after Noninfected Exogenous Injuries
Exogenous injuries to the tooth elicit the response of the local immune system of the pulpal tissue. The pulpal response directly involves various cell types that are distinctly regulated depending on the intensity and duration of these injuries. Thus, a sequence of biological mechanisms, such as apoptosis, cell proliferation, cell migration, and cell differentiation, is triggered in the pulpal tissue immediately after the injury, leading to proper healing of the dental pulp. The establishment of murine injury models without infection is advantageous to monitor the presence of DCs at the lesion site and their behavior after the injury. By excluding the influence of external harmful agents, such as bacteria and related substances, it is easier to analyze the influence of the local microenvironment for DC recruitment and their relationship to other pulpal cell populations.
The first study that explored the occurrence of pulpal MHC + class II cells under pathological conditions was published in 1991 by Bergenholtz et al. The dental pulp of rat incisors was exposed and irritated with bacteria-derived lipopolysaccharides and then covered with a temporal restoration. A significant increase in MHC + class II cells was observed in inflamed pulps compared to control. Cells presenting dendritic features were more abundant than those observed in the normal pulps of incisors [79]. Another similar study explored the response of immunocompetent cells in relation to neural elements using innervated or denervated pulps of rat molars. Four days after the operation, Ia + cells (a marker for immunocompetent cells) were densely distributed in the proximity of the injury in the innervated group, suggesting the importance of sensory nerves on the recruitment of immunocompetent cells [80].
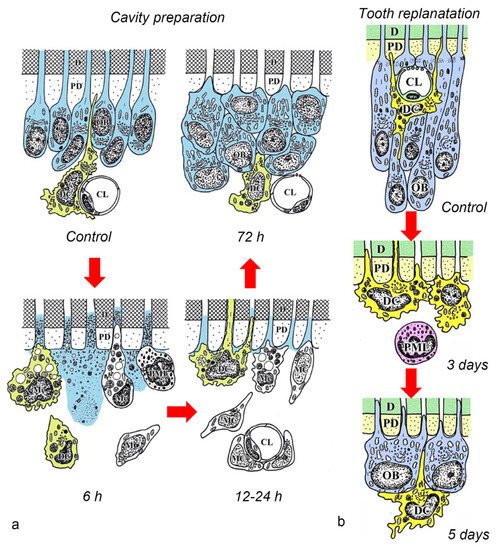
After the initial acute injury, exudative lesions appeared beneath the injury site and separated the few surviving odontoblasts from the predentin. MHC + class II cells, identified by the OX6 antibody, moved inward from the pulp-dentin border, and were relocated beneath the exudative lesions (asterisk) ( Figure 3 a) [40][44][81]. Six hours after the cavity preparation, the surviving odontoblasts began to degenerate and lost HSP-25 expression (a protein used as a rat odontoblast marker), whereas some of the OX6 + cells remained among them. Scattered OX6 + cells extended their dendritic process into the dentinal tubules. However, the number of these cells increased 12 h after the operation, especially under the afflicted area (arrow in Figure 3 b). Exudative lesions disappeared from 12 h to day 1 after the operation ( Figure 3 b). Odontoblasts did not show HSP-25 immunoreactivity. Moreover, when samples were analyzed by TEM, the cytoplasmic processes of OX6 + cells located deep into the dentinal tubules were characterized by tubulovesicular structures, multivesicular bodies, and vacuoles, as seen in DCs observed in other human tissue [40][44][81]. It is believed that pulpal macrophages and neutrophiles quickly remove the cell and dentinal debris induced by cavity preparation, thus facilitating the migration of DCs toward the pulp-dentin border [82]. Newly differentiated odontoblast-like cells began to appear 48 h after the initial injury. Although speculative, there is a possibility that HSP-25 can be discharged from dying odontoblasts to the extracellular milieu, where it could act as a chemotactic agent, favoring DC recruitment in the afflicted area [40][44][81]. Up to day 3 after the operation, the afflicted area of the pulp completely recovered, and newly differentiated odontoblast-like cells expressing HSP-25 aligned under predentin. OX6 + cells were located exclusively beneath the odontoblast layer ( Figure 1 c) and displayed the same ultrastructural features as those observed in control samples [40][44][81]. Previous findings were also confirmed in aged rat molars after the same injury model. Aged pulps still maintained a satisfactory self-defense capacity, with the same rate of recovery of younger pulps [47][83]. The summary of the morphological changes and the spatiotemporal relationship between odontoblasts and OX6 + cells in a rat model for cavity preparation are summarized in Figure 2 a.
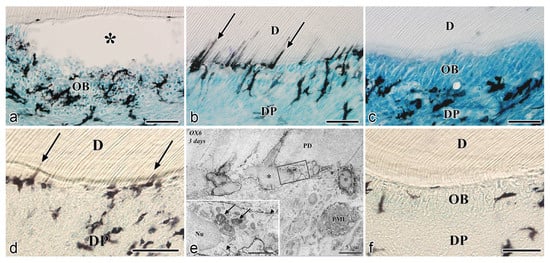
To analyze the behavior of DCs after severe injuries, another set of studies was carried out using the murine tooth replantation model. Rungvechvuttivittaya et al. investigated the kinetics of pulpal macrophages and MHC + class II cells after the tooth replantation. From days 3 to 7 after the operation, OX6 + cells with a dendritic profile concentrated in the pulp-dentin border areas where odontoblasts had died. Two weeks after the operation, a calcified tissue was observed under the predentin in all replanted teeth, indicating that the pulpal repair process had been completed [23][25]. Interestingly, ED1 + cells (a marker for macrophages) were seen in the same high proportion as OX6 + cells in cases where bone-like tissue was formed in the pulp of the replanted teeth [42]. Ohshima et al. confirmed the previous reports in two conclusive studies after the same methodology in rats. The activity of odontoblasts and odontoblast-like cells was related to the variation of OX6 + and ED1 + cell distribution in the dental pulp during its repair process. On day 1 after replantation, numerous polymorphonuclear leukocytes (PMLs) migrated through the damaged odontoblast layer, which lost HSP-25 immunoreactivity. OX6 + and ED1 + cells increased in number at the pulp periphery at this stage. On day 3, odontoblasts did not show HSP-25 immunoreactivity, nor major changes were noted in the number of OX6 + and ED1 + cells located at the pulp-dentin border (arrows) ( Figure 1 d). The ultrastructure of these cells presented the same tubulovesicular structures and multivesicular bodies in their cytoplasm as other pulpal DCs, whereas an intense immunoreactivity for OX6 was recognized in their cell membranes (arrows in inset) ( Figure 1 e). From days 3 to 5, OX6 + cells increased in number at the pulp periphery of replanted teeth. Newly differentiated odontoblast-like cells were identified on day 5 after replantation, showing intense HSP-25 immunoreactivity. Some OX6 + cells with dendritic profiles were observed beneath or in between them ( Figure 1 f). OX6 + cell accumulation was observed specifically in few areas where the pulpal repair process was still delayed. One week after replantation, reparative dentin was deposited by newly-differentiated odontoblast-like cells in most samples, and the number of OX6 + and ED1 + cells returned to normal. Scattered OX6 + cells remained at the pulp periphery and shifted their location to the subodontoblastic layer. From week 2 to up to 90 days, at least three distinct regeneration patterns were observed in the dental pulp of replanted teeth: reparative dentin, bone-like tissue, and a mixed form of reparative dentin and bone-like tissue. Interestingly, ED1 + cell aggregation and the appearance of abundant nerve fibers preceded the bone-like tissue healing pattern [43][45]. The spatiotemporal relationship between odontoblasts and OX6 + cells during the early stages of the pulpal defense response after the tooth replantation in rat molars is summarized in Figure 2 b.
4. Relationship between DCs and Stem/Progenitor Cells in the Dental Pulp after Injuries
Stem cells of the dental pulp are called DPSCs or, in immature teeth, stem cells from human exfoliated deciduous teeth. Other similar cells include dental pulp from the apical papilla and dental follicle progenitor cells. These cell populations have the capacity to differentiate into odontogenic cells and other cell lineages [7]. Bone marrow resident MSCs are located in perivascular areas of the bone marrow microenvironment, where they can be in close contact with immune cells, including B cells, T cells, and DCs [84][85]. Likewise, MSCs in the dental pulp harbor in perivascular niches, where they display a phenotype consistent with pericytes. It was proven that perivascular DPSCs are directly involved in the regulation of the tissue regeneration after mild or severe injuries, as in the formation of reactionary or reparative dentin [86][87][88]. Therefore, the ability of both young and old teeth to respond to injury by the induction of pathological dentinogenesis suggests that a small population of competent progenitor cells or pulp stem cells may exist within the dental pulp throughout life [7].
Previous in vitro experiments demonstrated that MSCs in a steady state exhibit inhibitory effects on DC differentiation, which resemble immature/semimature/tolerogenic DCs, even upon exposure to pro-maturation stimuli. In contrast, targeted downregulation of suppressive mechanisms by which MSCs act on DC differentiation and functions could break the immune tolerance in immunosuppressive environments such as tumors [89]. In addition, it has been proven that resting MSCs can further increase OPN production when cocultured with DCs. In contrast, in the presence of proinflammatory cytokines, MSCs exert an opposite effect inhibiting OPN production. OPN production by DCs and DC-conditioned medium enhances the osteogenic differentiation of MSCs, leading to the upregulation of the osteogenic markers alkaline phosphatase (ALP) and RUNX2 and the expression of the bone-anabolic chemokine CCL5. In contrast, OPN may play an inhibitory role in adipogenic differentiation. Thus, the interplay between DCs and MSCs may contribute to the upregulation of OPN production with the consequent inhibition of MSC-derived adipogenesis and the induction of osteogenic differentiation [84][90].
Although most evidence on the crosstalk between MSCs and DCs has been retrieved from nondental tissue, a previous study highlighted that periapical lesion-derived MSCs (PL-MSCs) could differentially modulate DC functions and Th-cell response in periodontal lesions. At the beginning of the disease, PL-MSCs could activate resident DCs, inducing their maturation and Th1 polarization. In contrast, in the later stages of PLs, PL-MSCs could potentiate DC tolerogenic differentiation and the polarization of immune response toward Th2 and Treg cells, which are important for the healing stage of periodontal lesions. Thus, this study showed that PL-MSCs could either augment the inflammatory response or contribute to the resolution of the disease, depending on the dynamics of the two cell types [89].
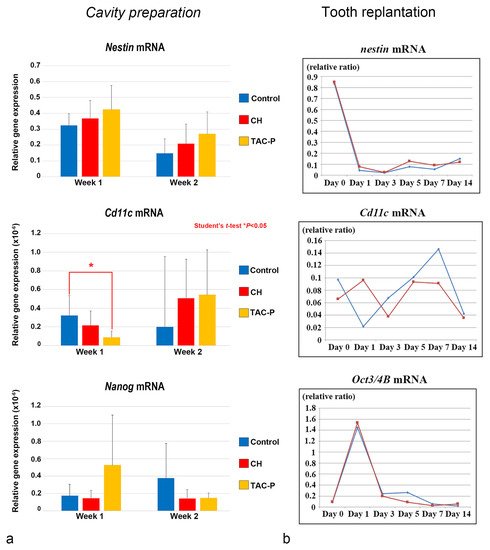
The relationship between DCs and DPSCs/progenitor cells during the healing process was evaluated in vivo in two different studies using cavity preparation and tooth replantation mouse models. The most recent study in this topic elucidated the responses of the oral microflora-exposed dental pulp to capping with a triple antibiotic paste (TAP: metronidazole, ciprofloxacin, and minocycline) in mouse molars, compared to those to calcium hydroxide (CH) cement, in addition to the combination of macrogol and propylene glycol (MP, control group), followed by a glass ionomer cement filling. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was performed using specific primers for cDNA encoding Nestin (odontoblast differentiation), Cd11c (DCs), and Nanog (stem/progenitor cells). Cd11c mRNA sharply increased from weeks 1 to 2 in the TAP group, followed by the CH group, and correlated with a peak in the cell proliferative activity. In contrast, the MP group markedly decreased its expression 2 weeks after treatment, with significant differences between the TAP and MP groups regarding the mRNA expression of Cd11c on week 1. mRNA expression of the odontoblast differentiation marker Nestin was higher in the TAP group than in the CH and MP groups at week 1, followed by a steady decrease of its expression in all groups on week 2. TAP group also showed the highest mRNA expression of the stem cell marker Nanog among the three groups on week 1, whereas its expression decreased by week 2. These results demonstrated that TAP might contribute to a sterile environment that allows active pulpal cell proliferation and the simultaneous activation of DCs. Moreover, high mRNA expression of the stem cell marker Nanog on week 1 after treatment suggested that the use of TAP favored the activation of stem cells/progenitors residing in the injured dental pulp ( Figure 7 a) [37]. Likewise, another study evaluated the effectiveness of the combination of antibacterial drugs to heal the dental pulp. The maxillary first molars of 3-week-old mice were extracted and immersed in the 3Mix solution for 30 within compared to phosphate buffered saline (PBS) alone. Although no significant difference was found between the 3Mix and PBS groups, the study showed chronological changes in the gene expression of differentiation markers such as Nestin , Dspp , Alp , Ocn , and Opn , and the evaluation of DC ( Cd11c ) and DPSC ( Oct3/4A and B ) activity during the pulpal healing process. High expression levels of Cd11c mRNA were first observed in the 3Mix group on day 1 and later on days 5 and 7, whereas, in the PBS group, these levels progressively increased from days 1 to 7. Moreover, the Oct3/4A and B transcript leves were greatly enhanced on day 1 in both groups, correlating with the increase of Cd11c mRNA in the 3Mix group ( Figure 3 b).
This entry is adapted from the peer-reviewed paper 10.3390/jcm10153348
References
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 9th ed.; Elsevier: St. Louis, MO, USA, 2018; p. 344.
- Berkovitz, B.K.B.; Holland, G.R.; Moxham, B.J.; Berkovitz, B.K.B. Oral Anatomy, Histology and Embryology, 5th ed.; Elsevier: Edinburgh, UK, 2018; pp. 1–462.
- Chiego, D.J. Essentials of Oral Histology and Embryology: A Clinical Approach, 5th ed.; Elsevier: St. Louis, MO, USA, 2018.
- Rajan, S.; Ljunggren, A.; Manton, D.J.; Bjorkner, A.E.; McCullough, M. Post-mitotic odontoblasts in health, disease, and regeneration. Arch. Oral. Biol. 2020, 109, 104591.
- Yumoto, H.; Hirao, K.; Hosokawa, Y.; Kuramoto, H.; Takegawa, D.; Nakanishi, T.; Matsuo, T. The roles of odontoblasts in dental pulp innate immunity. Jpn. Dent. Sci. Rev. 2018, 54, 105–117.
- Couve, E.; Osorio, R.; Schmachtenberg, O. The amazing odontoblast: Activity, autophagy, and aging. J. Dent. Res. 2013, 92, 765–772.
- Hargreaves, K.M.; Goodis, H.E.; Tay, F.R. Seltzer and Bender’s Dental Pulp, 2nd ed.; Quintessence Publishing: Hanover Park, IL, USA, 2012; p. 501.
- Orchardson, R.; Cadden, S.W. An update on the physiology of the dentine-pulp complex. Dent. Update 2001, 28, 200–209.
- Mjor, I.A.; Sveen, O.B.; Heyeraas, K.J. Pulp-dentin biology in restorative dentistry. Part 1: Normal structure and physiology. Quintessence Int. 2001, 32, 427–446.
- Casasco, A.; Casasco, M.; Calligaro, A.; Ferrieri, G.; Brambilla, E.; Strohmenger, L.; Alberici, R.; Mazzini, G. Cell proliferation in developing human dental pulp. A combined flow cytometric and immunohistochemical study. Eur. J. Oral Sci. 1997, 105, 609–613.
- Piattelli, A.; Rubini, C.; Fioroni, M.; Ciavarelli, L.; De Fazio, P. bcl-2, p53, and MIB-1 in human adult dental pulp. J. Endod. 2000, 26, 225–227.
- Vermelin, L.; Lecolle, S.; Septier, D.; Lasfargues, J.J.; Goldberg, M. Apoptosis in human and rat dental pulp. Eur. J. Oral Sci. 1996, 104, 547–553.
- Franquin, J.C.; Remusat, M.; Abou Hashieh, I.; Dejou, J. Immunocytochemical detection of apoptosis in human odontoblasts. Eur. J. Oral Sci. 1998, 106 (Suppl. 1), 384–387.
- Nishikawa, S.; Sasaki, F. Apoptosis of dental pulp cells and their elimination by macrophages and MHC class II-expressing dendritic cells. J. Histochem. Cytochem. 1999, 47, 303–312.
- Satchell, P.G.; Gutmann, J.L.; Witherspoon, D.E. Apoptosis: An introduction for the endodontist. Int. Endod. J. 2003, 36, 237–245.
- Harada, M.; Kenmotsu, S.; Nakasone, N.; Nakakura-Ohshima, K.; Ohshima, H. Cell dynamics in the pulpal healing process following cavity preparation in rat molars. Histochem. Cell Biol. 2008, 130, 773–783.
- Heyeraas, K.J.; Sveen, O.B.; Mjor, I.A. Pulp-dentin biology in restorative dentistry. Part 3: Pulpal inflammation and its sequelae. Quintessence Int. 2001, 32, 611–625.
- Bjorndal, L.; Mjor, I.A. Pulp-dentin biology in restorative dentistry. Part 4: Dental caries--characteristics of lesions and pulpal reactions. Quintessence Int. 2001, 32, 717–736.
- Mjor, I.A.; Odont, D. Pulp-dentin biology in restorative dentistry. Part 2: Initial reactions to preparation of teeth for restorative procedures. Quintessence Int. 2001, 32, 537–551.
- Farges, J.C.; Keller, J.F.; Carrouel, F.; Durand, S.H.; Romeas, A.; Bleicher, F.; Lebecque, S.; Staquet, M.J. Odontoblasts in the dental pulp immune response. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 425–436.
- Farges, J.C.; Alliot-Licht, B.; Baudouin, C.; Msika, P.; Bleicher, F.; Carrouel, F. Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front. Physiol. 2013, 4, 326.
- Farges, J.C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental pulp defence and repair mechanisms in dental caries. Mediat. Inflamm. 2015, 2015, 230251.
- Tziafas, D. Mechanisms controlling secondary initiation of dentinogenesis: A review. Int. Endod. J. 1994, 27, 61–74.
- Ruch, J.V.; Lesot, H.; Begue-Kirn, C. Odontoblast differentiation. Int. J. Dev. Biol. 1995, 39, 51–68.
- Smith, A.J.; Cassidy, N.; Perry, H.; Begue-Kirn, C.; Ruch, J.V.; Lesot, H. Reactionary dentinogenesis. Int. J. Dev. Biol. 1995, 39, 273–280.
- Magloire, H.; Romeas, A.; Melin, M.; Couble, M.L.; Bleicher, F.; Farges, J.C. Molecular regulation of odontoblast activity under dentin injury. Adv. Dent. Res. 2001, 15, 46–50.
- Smith, A.J.; Lesot, H. Induction and regulation of crown dentinogenesis: Embryonic events as a template for dental tissue repair? Crit. Rev. Oral Biol. Med. 2001, 12, 425–437.
- Tjaderhane, L. The mechanism of pulpal wound healing. Aust. Endod. J. 2002, 28, 68–74.
- Arana-Chavez, V.E.; Massa, L.F. Odontoblasts: The cells forming and maintaining dentine. Int. J. Biochem. Cell. Biol. 2004, 36, 1367–1373.
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin is essential for type I collagen secretion in reparative dentin. J. Dent. Res. 2016, 95, 1034–1041.
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth repair and regeneration. Curr. Oral Health Rep. 2018, 5, 295–303.
- Neves, V.C.M.; Sharpe, P.T. Regulation of reactionary dentine formation. J. Dent. Res. 2018, 97, 416–422.
- Tziafas, D. Characterization of odontoblast-like cell phenotype and reparative dentin formation in vivo: A comprehensive literature review. J. Endod. 2019, 45, 241–249.
- Dammaschke, T.; Stratmann, U.; Fischer, R.J.; Sagheri, D.; Schafer, E. Proliferation of rat molar pulp cells after direct pulp capping with dentine adhesive and calcium hydroxide. Clin. Oral Investig. 2011, 15, 577–587.
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Ohshima, H. Use of a triple antibiotic solution affects the healing process of intentionally delayed replanted teeth in mice. J. Oral Biosci. 2013, 55, 91–100.
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Ohshima, H. Effects of a triple antibiotic solution on pulpal dynamics after intentionally delayed tooth replantation in mice. J. Endod. 2014, 40, 1566–1572.
- Quispe-Salcedo, A.; Sato, T.; Matsuyama, J.; Ida-Yonemochi, H.; Ohshima, H. Responses of oral-microflora-exposed dental pulp to capping with a triple antibiotic paste or calcium hydroxide cement in mouse molars. Regen. Ther. 2020, 15, 216–225.
- Saito, K.; Nakatomi, M.; Ohshima, H. Dynamics of bromodeoxyuridine label-retaining dental pulp cells during pulpal healing after cavity preparation in mice. J. Endod. 2013, 39, 1250–1255.
- Ohshima, H.; Kawahara, I.; Maeda, T.; Takano, Y. The relationship between odontoblasts and immunocompetent cells during dentinogenesis in rat incisors: An immunohistochemical study using OX6-monoclonal antibody. Arch. Histol. Cytol. 1994, 57, 435–447.
- Ohshima, H.; Sato, O.; Kawahara, I.; Maeda, T.; Takano, Y. Responses of immunocompetent cells to cavity preparation in rat molars: An immunohistochemical study using OX6-monoclonal antibody. Connect. Tissue Res. 1995, 32, 303–311.
- Kannari, N.; Ohshima, H.; Maeda, T.; Noda, T.; Takano, Y. Class II MHC antigen-expressing cells in the pulp tissue of human deciduous teeth prior to shedding. Arch. Histol. Cytol. 1998, 61, 1–15.
- Rungvechvuttivittaya, S.; Okiji, T.; Suda, H. Responses of macrophage-associated antigen-expressing cells in the dental pulp of rat molars to experimental tooth replantation. Arch. Oral Biol. 1998, 43, 701–710.
- Shimizu, A.; Nakakura-Ohshima, K.; Noda, T.; Maeda, T.; Ohshima, H. Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars. Cell Tissue Res. 2000, 302, 221–233.
- Ohshima, H.; Nakakura-Ohshima, K.; Takeuchi, K.; Hoshino, M.; Takano, Y.; Maeda, T. Pulpal regeneration after cavity preparation, with special reference to close spatio-relationships between odontoblasts and immunocompetent cells. Microsc. Res. Tech. 2003, 60, 483–490.
- Nakakura-Ohshima, K.; Watanabe, J.; Kenmotsu, S.; Ohshima, H. Possible role of immunocompetent cells and the expression of heat shock protein-25 in the process of pulpal regeneration after tooth injury in rat molars. J. Electron. Microsc. 2003, 52, 581–591.
- Suzuki, T.; Nomura, S.; Maeda, T.; Ohshima, H. An immunocytochemical study of pulpal responses to cavity preparation by laser ablation in rat molars by using antibodies to heat shock protein (Hsp) 25 and class II MHC antigen. Cell Tissue Res. 2004, 315, 311–319.
- Kawagishi, E.; Nakakura-Ohshima, K.; Nomura, S.; Ohshima, H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006, 326, 111–122.
- Sato, T.; Kenmotsu, S.; Nakakura-Ohshima, K.; Takahashi, N.; Ohshima, H. Responses of infected dental pulp to α TCP-containing antimicrobials in rat molars. Arch. Histol. Cytol. 2011, 73, 165–175.
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Kenmotsu, S.; Ohshima, H. The expression of GM-CSF and osteopontin in immunocompetent cells precedes the odontoblast differentiation following allogenic tooth transplantation in mice. J. Histochem. Cytochem. 2011, 59, 518–529.
- Ohshima, H.; Maeda, T.; Takano, Y. Acid phosphatase activity in the class II MHC antigen-expressing cells in the rat incisor pulp. Dent. Jpn. 1997, 33, 8–14.
- Ohshima, H.; Takano, Y.; Sato, O.; Kawahara, I.; Maeda, T. Responses of class II MHC antigen-expressing cells to cavity preparation. In Dentin/Pulp Complex, Proceedings of the International Conference on Dentin Pulp Complex, Chiba, Japan, 1-4 July 1995; Quintessence Pub. Co.: Tokyo, Japan; Chicago, IL, USA, 1996; pp. 316–318.
- Lhuillier, C.; Galluzzi, L. Preface—Dendritic cells: Master regulators of innate and adaptive immunity. Int. Rev. Cell. Mol. Biol. 2019, 348, ix–xiv.
- Devi, K.S.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2017, 39, 137–152.
- Tsukamoto-Tanaka, H.; Ikegame, M.; Takagi, R.; Harada, H.; Ohshima, H. Histochemical and immunocytochemical study of hard tissue formation in dental pulp during the healing process in rat molars after tooth replantation. Cell Tissue Res. 2006, 325, 219–229.
- Ogawa, R.; Saito, C.; Jung, H.S.; Ohshima, H. Capacity of dental pulp differentiation after tooth transplantation. Cell Tissue Res. 2006, 326, 715–724.
- Takamori, Y.; Suzuki, H.; Nakakura-Ohshima, K.; Cai, J.; Cho, S.W.; Jung, H.S.; Ohshima, H. Capacity of dental pulp differentiation in mouse molars as demonstrated by allogenic tooth transplantation. J. Histochem. Cytochem. 2008, 56, 1075–1086.
- Unno, H.; Suzuki, H.; Nakakura-Ohshima, K.; Jung, H.S.; Ohshima, H. Pulpal regeneration following allogenic tooth transplantation into mouse maxilla. Anat. Rec. 2009, 292, 570–579.
- Mutoh, N.; Nakatomi, M.; Ida-Yonemochi, H.; Nakagawa, E.; Tani-Ishii, N.; Ohshima, H. Responses of BrdU label-retaining dental pulp cells to allogenic tooth transplantation into mouse maxilla. Histochem. Cell Biol. 2011, 136, 649–661.
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Ohshima, H. The effects of enzymatically synthesized glycogen on the pulpal healing process of extracted teeth following intentionally delayed replantation in mice. J. Oral Biosci. 2015, 57, 124–130.
- Kuratate, M.; Yoshiba, K.; Shigetani, Y.; Yoshiba, N.; Ohshima, H.; Okiji, T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. J. Endod. 2008, 34, 970–974.
- Shigetani, Y.; Suzuki, H.; Ohshima, H.; Yoshiba, K.; Yoshiba, N.; Okiji, T. Odontoblast response to cavity preparation with Er:YAG laser in rat molars: An immunohistochemical study. Odontology 2013, 101, 186–192.
- Nakakura-Ohshima, K.; Quispe-Salcedo, A.; Sano, H.; Hayasaki, H.; Ohshima, H. The effects of reducing the root length by apicoectomy on dental pulp revascularization following tooth replantation in mice. Dent. Traumatol. 2021.
- Ishikawa, Y.; Ida-Yonemochi, H.; Nakakura-Ohshima, K.; Ohshima, H. The relationship between cell proliferation and differentiation and mapping of putative dental pulp stem/progenitor cells during mouse molar development by chasing BrdU-labeling. Cell Tissue Res. 2012, 348, 95–107.
- Ishikawa, Y.; Ida-Yonemochi, H.; Suzuki, H.; Nakakura-Ohshima, K.; Jung, H.S.; Honda, M.J.; Ishii, Y.; Watanabe, N.; Ohshima, H. Mapping of BrdU label-retaining dental pulp cells in growing teeth and their regenerative capacity after injuries. Histochem. Cell Biol. 2010, 134, 227–241.
- Saito, K.; Ishikawa, Y.; Nakakura-Ohshima, K.; Ida-Yonemochi, H.; Nakatomi, M.; Kenmotsu, S.; Ohshima, H. Differentiation capacity of BrdU label-retaining dental pulp cells during pulpal healing following allogenic transplantation in mice. Biomed. Res. 2011, 32, 247–257.
- Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab. Anim. 2010, 44, 1–6.
- Andreasen, J.O.; Andersson, L. Critical considerations when planning experimental in vivo studies in dental traumatology. Dent. Traumatol. 2011, 27, 275–280.
- Kim, S.; Shin, S.J.; Song, Y.; Kim, E. In vivo experiments with dental pulp stem cells for pulp-dentin complex regeneration. Mediat. Inflamm. 2015, 2015, 409347.
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell. Mol. Biol. 2019, 348, 1–68.
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162.
- Patel, V.I.; Metcalf, J.P. Identification and characterization of human dendritic cell subsets in the steady state: A review of our current knowledge. J. Investig. Med. 2016, 64, 833–847.
- Nudel, I.; Elnekave, M.; Furmanov, K.; Arizon, M.; Clausen, B.E.; Wilensky, A.; Hovav, A.H. Dendritic cells in distinct oral mucosal tissues engage different mechanisms to prime CD8+ T cells. J. Immunol. 2011, 186, 891–900.
- Geginat, J.; Nizzoli, G.; Paroni, M.; Maglie, S.; Larghi, P.; Pascolo, S.; Abrignani, S. Immunity to pathogens taught by specialized human dendritic cell subsets. Front. Immunol. 2015, 6, 527.
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20.
- Constantino, J.; Gomes, C.; Falcao, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017, 65, 798–810.
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis, E.S.C. Dendritic cells revisited. Annu. Rev. Immunol. 2021, 39, 131–166.
- Keselowsky, B.G.; Lewis, J.S. Dendritic cells in the host response to implanted materials. Semin. Immunol. 2017, 29, 33–40.
- Van Brussel, I.; Berneman, Z.N.; Cools, N. Optimizing dendritic cell-based immunotherapy: Tackling the complexity of different arms of the immune system. Mediat. Inflamm. 2012, 2012, 690643.
- Bergenholtz, G.; Nagaoka, S.; Jontell, M. Class II antigen expressing cells in experimentally induced pulpitis. Int. Endod. J. 1991, 24, 8–14.
- Fristad, I.; Heyeraas, K.J.; Kvinnsland, I.H.; Jonsson, R. Recruitment of immunocompetent cells after dentinal injuries in innervated and denervated young rat molars: An immunohistochemical study. J. Histochem. Cytochem. 1995, 43, 871–879.
- Ohshima, H.; Nakakura-Ohshima, K.; Maeda, T. Expression of heat-shock protein 25 immunoreactivity in the dental pulp and enamel organ during odontogenesis in the rat molar. Connect. Tissue Res. 2002, 43, 220–223.
- Kitamura, C.; Kimura, K.; Nakayama, T.; Toyoshima, K.; Terashita, M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. J. Dent. Res. 2001, 80, 1530–1534.
- Izumi, T.; Inoue, H.; Matsuura, H.; Mukae, F.; Ishikawa, H.; Hirano, H.; Tamura, N. Age-related changes in the immunoreactivity of the monocyte/macrophage system in rat molar pulp after cavity preparation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 103–110.
- Del Prete, A.; Scutera, S.; Sozzani, S.; Musso, T. Role of osteopontin in dendritic cell shaping of immune responses. Cytokine Growth Factor Rev. 2019, 50, 19–28.
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131.
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704.
- Pang, Y.W.; Feng, J.; Daltoe, F.; Fatscher, R.; Gentleman, E.; Gentleman, M.M.; Sharpe, P.T. Perivascular stem cells at the tip of mouse incisors regulate tissue regeneration. J. Bone Miner. Res. 2016, 31, 514–523.
- Vidovic-Zdrilic, I.; Vijaykumar, A.; Mina, M. Activation of alphaSMA expressing perivascular cells during reactionary dentinogenesis. Int. Endod. J. 2019, 52, 68–76.
- Ethokic, J.M.; Tomic, S.Z.; Colic, M.J. Cross-talk between mesenchymal stem/stromal cells and dendritic cells. Curr. Stem Cell Res. Ther. 2016, 11, 51–65.
- Scutera, S.; Salvi, V.; Lorenzi, L.; Piersigilli, G.; Lonardi, S.; Alotto, D.; Casarin, S.; Castagnoli, C.; Dander, E.; D’Amico, G.; et al. Adaptive regulation of osteopontin production by dendritic cells through the bidirectional interaction with mesenchymal stromal cells. Front. Immunol. 2018, 9, 1207.
