The wide variety of epigenetic controls available is rapidly expanding the knowledge of molecular biology even overflowing it. At the same time, it can illuminate unsuspected ways of understanding the etiology of cancer. New emerging therapeutic horizons, then, promise to overcome the current antitumor strategies need. The translational utility of this complexity is particularly welcome in oral cancer (OC), in which natural history is alarmingly disappointing due to the invasive and mutilating surgery, the high relapsing rate, the poor quality of life and the reduced survival after diagnosis. Melatonin activates protective receptor-dependent and receptor-independent processes that prevent tissue cancerisation and inhibit progressive tumor malignancy and metastasis.
1. Introduction
The oral cavity is the anatomical place where approximately 50% of head and neck cancers (HNC) appear. Oral cancer (OC) can originate in the epithelium covering any anatomical structure of the oropharyngeal and nasal cavities. The oral squamous cell carcinomas (OSCC) and oropharynx mucosa, also known as epidermoid oral carcinoma, are the most frequent malignant diseases of the upper aerodigestive tract, representing 90–95% of new diagnoses of oral malignancies [
1].
The poor prognosis and high mortality that still characterize OC, combined with the lack of better future perspectives, is a challenging panorama. The unreliability of screening tests or sensitive biomarkers for a precise diagnosis, together with the unavailability of effective therapeutic tools hampering malignant progression and drug resistance, add concern to the current situation. In this regard, the clinical outcome of OC patients is unpredictable and life threatening, leading to unsatisfactory 5-year survival rates (~50%), which has remained unchanged in recent decades [
2,
3]. This unfavorable clinical prognosis results from the high tendency of oral tumors to proliferate rapidly, doubling in size every ~6–7 days [
4], the development of locoregional recurrence, second primary tumors and radiochemotherapy resistance. As a result, the tumor cells spread out to secondary tissues (mainly the lungs, liver and bones) [
3], diminishing the survival of OC patients [
3].
Up to an 80% of OC patients display unpredictable and erratic outcomes in response to radiochemotherapy, which is related to genetic variability. The heterogeneous spectrum of interindividual epigenetic changes [
5] makes the assessment and therapeutic management of this disease difficult. Therefore, the knowledge of the epigenetic pathways behind oral malignancy would offer a great opportunity for personalized treatments and patient follow-up [
6]. In this regard, the large amounts of data obtained from current molecular technologies facilitates the design of an OC meta-signature based on its epigenetic features [
7]. It is expected that in this way a new generation of biomarkers will allow improved early and highly predictive diagnoses, enabling tailored therapies directed to the epigenetic idiosyncrasy of each tumor [
8]. Consequently, the development of robust multiplexing predictors may potentiate the clinical outcome and quality of life of OC patients [
9]. The understanding of the epigenetic map of OC is therefore a necessary step to make the clinical practice more effective, allowing it to evolve from histopathology to a molecular-based era [
10].
In this complex scenario, we will focus special attention on the role of melatonin as an adjuvant treatment in OC therapy. Although under physiological conditions melatonin is mainly released from the pineal gland, other significant extrapineal sources of melatonin such as the enteroendocrine cells of the gastrointestinal tract, including the oral cavity and the salivary glands should be considered too [
11]. Albumin-free blood melatonin passively diffuses via saliva into the mouth, where it reaches amounts 70% lower than in circulation [
12]. In vertebrates salivary melatonin levels oscillate according to the rhythmicity observed in the serum [
13], from a diurnal 1–5 pg/mL to 50 pg/mL at night [
12]. Saliva is acquiring a great deal of attention as a preferential milieu to search for OC biomarkers [
14,
15]. Simultaneously, it is an excellent route for the direct administration of melatonin, alone or combined with standard therapeutic strategies. In this regard, melatonin enhances radiochemotherapy cytotoxicity by stimulating intracellular generation and accumulation of reactive oxygen species (ROS), apoptosis and autophagy [
16,
17], while in parallel melatonin antagonizes several undesired side effects on the oral mucosa [
18,
19]. It is of great translational importance to undertake blinded, independent and randomized controlled trials that corroborate the apparent protective effects on the oral cavity of local and systemic melatonin. The lack of toxicity and side effects reported by the different studies conducted in vivo, as well as the amelioration of life quality and survival of OC patients urge those melatonin studies. Last but not least, melatonergic treatments are inexpensive when compared to the standard radiochemotherapy currently in use, and this reinforces the necessity to deeply explore the clinical utility of this indoleamine.
2. The Role of Melatonin in the Oral Cavity: Functionality and Alterations in Oral Cancer
The beneficial role of melatonin for the management of many cancers has been widely proposed [
36,
37,
38]. However, the potential benefits of melatonin in the treatment of oral carcinomas are less well known and it is therefore, imperative to concentrate research in this area.
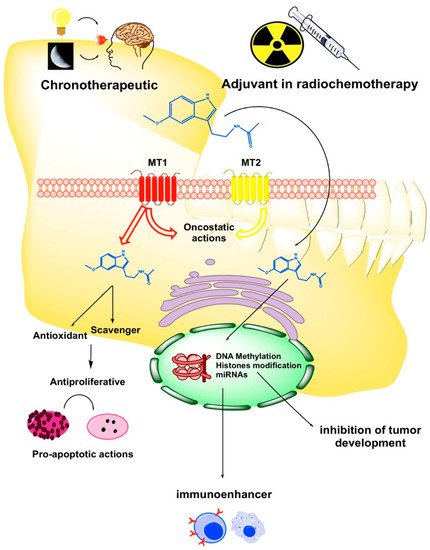
Oxidative stress (OS) and ROS generation are two pivotal factors involved in oral cancerisation through: (i) The damage of the DNA and proteins of normal keratinocytes; (ii) the oncogenic mutations and malignant transformation of oropharyngeal mucosa and (iii) the enhancement of progression and invasiveness of OC cells. In this regard, melatonin displays oncoprotective and oncostatic activities due to its antioxidant properties as a powerful scavenger of ROS [
39] (
Figure 1). Under hypoxic conditions tumor cells express the hypoxia inductive factor 1-alpha (HIF-1α), which induces the overexpression of pro-angiogenic vascular endothelial growth factor (VEGF) involved in tumor progression and metastases [
40]. In this context, melatonin strongly inhibits VEGF and other stress factors such as epidermal growth factor (EGF) or insulin growth factor-1 (IGF-1) [
40]. Thus, melatonin interferes with tumoral cell proliferation and tumor growth and acts as a pro-apoptotic agent in tumoral keratinocytes [
39,
41]. Proof of the anti-tumor activity of melatonin has been recently reported citing the association of the melatonin synthesis/metabolism index in the tumor microenvironment, with reduced somatic mutations and neoantigen expression in head and neck squamous carcinomas [
42].
Figure 1. Schematic diagram of the critical points where melatonin may exert its protective and therapeutic effects on oral cancer (OC). MT1: melatonin receptor type 1; MT2: melatonin receptor type 2.
The innate and adaptative immune cells are efficient cytotoxic anti-tumor defenses. However, eventually tumor cells evade immune surveillance by controlling cellular apoptosis and initiate the tumor cycle that leads to the development of cancer and tumor progression. In this regard, it has been described a cell-mediated immune depression in HNC, even after surgical resection [
43]. Melatonin is a potent immunoenhancer that increases the activity of T and B lymphocytes, monocytes and natural killer (NK) cells and stimulates the secretion of cytokines (interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α)) (
Figure 1), thereby leading to oncostasis and the inhibition of tumor spreading [
24].
3. Epigenetic Regulation of Melatonin in Oral Cancer
There is not a unified etiological model accounting for all diagnosed OC cases [
59]. In contrast, molecular defects on oncogenes and tumor suppressors that lead to genetic reprogramming of OC had been recently extended with the epigenetic aberrations in key homeostatic genes. Specifically, a mechanistic triad integrated by chemical modifications of DNA and associated proteins, in addition to non-coding RNAs inscribes heritable changes in the genome without affecting the coding sequence [
60]. Thereby epigenetic marks modulate genome transcription and control some relevant cancer hallmarks. Melatonin is also in focus as a likely relevant mediator of epigenetic effects on OC cells (Table 3).
3.1. Epigenetic Methylation of DNA
Even though OC epigenomics is still in its infancy, the carcinogenic importance of impairing certain tumor suppressors by an extensive promoter CpG methylation has increased incessantly in recent years. Cellular and molecular evidence from OSCC have proven that transcriptional silencing is as relevant in halting gene expression as the classical point mutations or the loss of heterozygosity [
59,
61,
62]. In this regard, putative suppressors are consistently active in healthy oral mucosa, while they are functionally cancelled in OSCC lines or malignant tissue either by double deletion or epigenetic silencing by abnormal DNA hypermethylation. The confidence in the clinical utility as a tumor biomarker of the gene-methylation profiling (hypermethylome) has spurred research and made it possible to identify additional accredited or presumed tumor suppressors involved in cell homeostasis and gene methylation maintenance. In the search for switched-off suppressors, the detailed examination of OSCC lines for bi-allelic deleted regions found a common ~1 Mb genetic loss at 4q35 where the melatonin receptor 1A gene is mapped (Table 3), thus suggesting MT1 as a new candidate for tumor suppressor in OSCC. Accordingly, the mRNA for MT1 was consistently lost or reduced in deleted lines, while in non-homozygously deleted tumor lines the MT1 promoter hypermethylation was inversely correlated with MT1 expression [
50]. Moreover, the tissue from primary OSCC tumors showed that the methylated promoter MT1 and the lack of MT1 were significantly associated with poor prognosis. All the above suggests that i) OC methylotype shows specific altered DNA methylation patterns in the course of tumor initiation and progression [
59]; ii) the promoter hypermethylation is a leading cause of tumor suppressors’ inhibition in OC pathogenesis [
63]; and iii) the loss of function of the high affinity MT1 may be pivotal in the etiopathogenesis of OC. However, research has also reported that the global OC genome is hypomethylated and increasingly hypomethylated in univariate association with tobacco/alcohol history and the malignant stage of the tumor [
64,
65]. Nevertheless, the individual DNA methylotype maintains translational interest for molecular staging, prognosis and customized management of OC patients [
66,
67,
68,
69]. In support of this are the recent results obtained by connecting the alteration of the DNA methylotype of HNC patients with gene expression profiling to screen their biomarker potency [
70,
71]. Similarly, LINE-1 hypomethylation in peripheral blood mononuclear cells (PBMCs) when compared to control PBMCs has recently proved to be an excellent OC diagnostic biomarker of maximum sensitivity and specificity [
72].
Light pollution at night (light-at-night or LAN) has recently provided evidence for the potential to facilitate cancer. Prominently, LAN alters the quality of sleep and presumably activates some putative mechanisms underlying malignant transformation, including the modification of global DNA methylation through pineal melatonin [
73]. In human oral mucosa is critical the melatonin-dependent and one-day rhythmic expression of the clock gene and tumor-suppressor
PER2, as well as its rapid adaptation to wavelength changes [
74]. Therefore, as it is known that circadian alterations promote some prevalent neoplasias [
75], that chronodisruption induces epigenetic abnormalities [
76] and that melatonin counteracts the carcinogenic effects of circadian impairments [
77,
78,
79], the time has now come to undertake a rigorous study of the circadian machinery and melatonin chronomodulation in OC (
Figure 1). The mechanism of how melatonin contributes to the epigenetic remodelling of methylome in OC cells requires a deeper research. In this regard, it is admissible that melatonin participates in the reversal of epigenetic impairments that deteriorate physiological transcription during oral malignisation and OC development.
Due to its magnitude, dynamic nature and variability of regulatory mechanisms DNA methylation adds much complexity to the molecular etiology of OC. DNA methylotype is extensive, because it affects a large number of genes (around 1300 according to estimates of a recent survey) [
80], dynamic, because the panel of methylated genes in primary oral tumors does not match that found in the metastases of the same patients [
78] and variable because significant differences are detected in the interindividual DNA methylation phenotype of OC patients [
59]. Particularly in OC, the dynamic and variable character of gene methylation makes it necessary to screen the methylome from tumor and paired-healthy tissue specimens, because certain habits such as smoking have been shown to increase DNA methylation of oral mucosa in non-negligible percentages [
81]. Moreover, the standardization of methylotype from tumor and matched-healthy tissue genotyping can help to prevent the other debated inconsistencies, such as the upregulated DNA methylation found in premalignant oral lesions [
82] and transitional tissue [
83], pointing out that epigenetic disturbances presumably occur in the early phases of OC carcinogenesis.
3.2. Epigenetic Modification of Chromatin Structure
The catalogue of epigenetic mechanisms set in motion by melatonin is not limited to promoter hypermethylation; chemical modifications of chromatin-associated proteins respond to the same mode of controlling gene expression with no alteration in the base-pair sequence of DNA. This is the case of lysine-specific histone acetylation/deacetylation within the N-terminus protruding from the nucleosome core, that switches chromatin’s structure between the permissive (acetylated), and the highly condensed repressive (deacetylated) transcriptional states [
84]. In this regard, hypoacetylation of acetyl-histone H3 at Lys9 has been associated with oral cancerisation and the poor prognosis of OC patients [
85]. For the same reason, an inadequate activation of histone lysine-specific demethylase (LSD1) enhanced cancer development and worsened the prognosis of tongue squamous cell carcinoma [
86]. Recently, LSD1 has been found upregulated in both clinical OSCC samples and null mice xenografted with implants from lymphoid metastatic OSSC patients [
87] and LSD1 overexpression correlated with negative cancer evolution [
86,
87]. In congruence with this evidence, the inhibition or repression of tumor promoting LSD1 brings about histone methylation and can lead to cancer arrest. Of note, melatonin has demonstrated in a mouse preclinical model of patient-derived OSCC xenografts that it has the potential to arrest cell cycle (at G0/G1 phase) and inhibit tumor proliferation by inducing histone H3 acetylation at Lys 4 and 9 through LSD1 suppression in both tongue and gingival cancer cells [
87] (Table 3). It is highly remarkable that melatonin showed this oncostatic potential on patient-derived xenografts to a similar extent as 5-FU, but is devoid of any undesirable cardiotoxic side effects. Likewise, the inhibition of histone deacetylase induced by melatonin has revealed to be efficient in reducing the radiotherapy-induced mucositis in radiated hamsters [
88], as well as in patients affected by OC and other head and neck carcinomas receiving concurrent chemoradiation [
58,
89], which reinforces the pleiotropic benefits of melatonin administration.
In HSC-3 and OECM-1 OC cells treated with melatonin (0–1 mM) for 24 h suppressed the expression and activation of proteolytic matrix metalloproteinase 9 (MMP-9) throughout the downregulation of extracellular signal-regulated 1 and 2 (ERK1/2) mitogen-activated protein (MAP) kinases kinases, which induced the reduction of transcriptional coactivators CREBBP/EP300 and thereby the depletion of active pool of MMP-9 by attenuating histone acetylation [
90] (Table 3). This process abrogates the active transcriptional complexes on MMP-9 promoter-deprived OC cells of this crucial extracellular matrix-degrading enzyme and consequently attenuates the propagative and metastatic capacities of OC tumors. Consistent with the foregoing, MMP-9 and CREBBP/EP300 are significantly overexpressed in the tumor tissue of paired tumor/non-tumor OC surgical specimens [
90]. In a similar way, millimolar melatonin reduced the migration and metastatic invasion of nasopharyngeal carcinoma cells through the suppression of MMP-9 expression by inhibiting the binding of the transcription factor, specificity protein-1 (SP-1) to DNA at the MMP-9 promoter [
91] (Table 3). Thus, another epigenetic action of melatonin in OC is the protection of the internal environment of oral tissues, which enzymatic destruction precedes the departure of metastatic cells.
3.3. Non-Coding micro-RNAs
The triggering of OC pathogenesis may include the dysregulation of part of the ~2500 human [
15] non-coding short (~22 nt) micro-RNAs (miRNAs), which bind complimentary target mRNAs and initiate their degradation or repression via RNA interference. Directly or indirectly, it is estimated that each miRNA can modulate thousands of genes through negative translational regulation [
92], so that expressed miRNAs are indicative of the functional profile of the cell. It is thus plausible that miRNAs can modulate crucial pro- and anticarcinogenic genes with oncogenic or suppressive roles in early OC [
93]. Given that precursor and mature miRNA expression is tumor and tissue specific, the numerous studies that have been undertaken during the last few years have revealed multiple altered miRNAs in oral diseases, premalignant lesions and OSCC [
15,
94,
95,
96]. Deregulated miRNAs in OC include several dozens of up-regulated oncogenic miRNAs (OncomiRs) and depleted miRNAs oncosuppressors [
95], eventually within wide dynamic ranges of variation. More importantly, the unveiled association with development, clinicopathological features, progression and/or therapy resistance of oral tumors has led to the proposal that some miRNAs are putative predictors of early carcinogenesis and outcome of OC patients [
95,
97].
The impact of melatonin on miRNA expression in paired healthy/tumor OC tissue has been scarcely addressed (
Table 3). Attention has been recently focused on oncogenic miRNA-24, up-regulated in OSCC cells with respect to normal keratinocytes, in tumor tissues of the oral cavity relative to healthy ones, as well as in the plasma of OSCC patients [
98]. On the other hand, in MCF-7 cells, 22 miRNAs differentially modulated by physiological nanomolar melatonin [
99], including down-regulated miRNA-24 [
100], may be held partially responsible for the antitumor effects of the indoleamine [
101]. In this regard, micromolar melatonin induced the repression of miRNA-24 and almost reversed the activation of a four-gene panel shared by melatonin/miRNA-24 pathways [
100], which aided the inhibition of tumor cell proliferation and the migration of colon, breast and head/neck tumor cells. It is therefore of great interest to investigate the effect of melatonin on miRNA-24 in the tumoral phenotype of OC cells.
Table 3. Studies supporting the use of melatonin on epigenetic modulation of OC.
| Epigenetic Control |
Experimental Model |
Melatonin Treatment |
Main Findings |
References |
| DNA methylation |
OSCC cell lines |
|
The loss by homozygous deletion or silencing by CpG hypermethylation of the MLT receptor 1A (MTNR1A) gene was associated with cancer status and tumor phenotype |
[50] |
| Histone modification |
Patient-derived tumor xenografts models overexpressing LSD1. Mouse-based subcutaneous OC SCC25-xenograft model.
OSCC cell lines. |
20 mg/kg daily, i.p., for 24 and 42 days.
0–20 mM for 24 h
2–4 mM for 24 and 48 h |
MLT demonstrated anti-OC activity through LSD1 down-regulation |
[87] |
| |
HSC-3 and OECM-1 OC cell lines |
1 mM for 24 h |
MLT inhibited migration of tumor cells through down-regulation of MMP-9 expression and activity by decreasing CREBBP/EP300-dependent H3 and H4 histone acetylation on MMP-9 promoter |
[90] |
| Promoter activity |
HONE-1, NPC-39 and NPC-BM nasopharyngeal carcinoma cell lines. |
0.5–1 mM |
MLT reduced MMP-9 promoter activity through inhibition of SP-1 transcription factor expression |
[91] |
| |
SCC9, SCC25 and CAL27 OSCC cell lines. |
10 μg/mL for 72 h |
MLT reduced miR-155 and increased miR-21. |
[102] |
| Non-coding micro-RNAs |
121 OC specimens and 66 normal counterparts for the study of miR-24 expression
HCT 116 and MCF-7 cells. |
1 μM for 72 h |
MLT decreased miR-24 expression, which pairs with the regulation of cell proliferation, DNA damage and oncogenic transformation genes. |
[100] |
Other miRNAs targeted by melatonin in OC have recently been identified in the baseline content of exosomes from SCC9, SCC25 and CAL27 lines [
102]. As previously performed using tumor lines profiled for expressed miRNAs [
103], this pioneering study described the exosomal signature of miRNA secreted by different OC cells and their different modulation by micromolar melatonin for 72 h. Specifically, the expression of miR-155 dropped while the presence of miR-21 raised in all the exosome fractions analyzed. miR-21 is a recognized OncomiR in OC [
104,
105,
106,
107], which is highly overexpressed in tumor lines [
108] and tissue samples [
109] and associated with a negative prognosis in OC patients [
110]. In this context, it has not only been reported that miR-21 is overexpressed in direct exfoliation cells of tumor foci [
109] and in tissue specimens and oral swirls [
111] of both OC patients and the controls, but also, this up-regulation has been directly associated with the cancerisation of oral tissues [
109]. The latest report of up-regulated miR-21 was in the exosomal content of OC lines exposed to melatonin is surprising [
102]. Certainly, gene and miRNA expression are under the control of multiple mediators and melatonin could be only one of the actors modulating the expression of miR-21. Nevertheless, the apparent influence of melatonin on miR-21 expression in the exosomes emitted by tumor cells of oral cavity highlights the active role that melatonin may play in the control of oral carcinogenesis.
The panel of genes epigenetically targeted by melatonin have a high translational value for OC therapy although they remain virtually unexplored. For example, their biological potential as early reporters of OC pathogenicity of paracrine miRNAs originating from damaged cells, or from active exosomal secretion is extraordinary, but unfortunately, the influence of pleiotropic melatonin on their functional biology has not been studied in depth. Nonetheless, the incentive of easily assessable biomarkers on read-out platforms catalyses the ongoing efforts to setting miRNAs and remaining epigenetic marks on specific palettes, for the screening of diagnosis, prognosis and personalized therapy of OC.
This entry is adapted from the peer-reviewed paper 10.3390/cancers11111712