para-Selective processes for the chlorination of phenols using sulphuryl chloride in the presence of various sulphur-containing catalysts have been successfully developed. Several chlorinated phenols, especially those derived by para-chlorination of phenol, ortho-cresol, meta-cresol, and meta-xylenol, are of significant commercial importance, but chlorination reactions of such phenols are not always as regioselective as would be desirable. We, therefore, undertook the challenge of developing suitable catalysts that might promote greater regioselectivity under conditions that might still be applicable for the commercial manufacture of products on a large scale. In this review, we chart our progress in this endeavour from early studies involving inorganic solids as potential catalysts, through the use of simple dialkyl sulphides, which were effective but unsuitable for commercial application, and through a variety of other types of sulphur compounds, to the eventual identification of particular poly(alkylene sulphide)s as very useful catalysts. When used in conjunction with a Lewis acid such as aluminium or ferric chloride as an activator, and with sulphuryl chloride as the reagent, quantitative yields of chlorophenols can be obtained with very high regioselectivity in the presence of tiny amounts of the polymeric sulphides, usually in solvent-free conditions (unless the phenol starting material is solid at temperatures even above about 50 °C). Notably, poly(alkylene sulphide)s containing longer spacer groups are particularly para-selective in the chlorination of m-cresol and m-xylenol, while, ones with shorter spacers are particularly para-selective in the chlorination of phenol, 2-chlorophenol, and o-cresol. Such chlorination processes result in some of the highest para/ortho ratios reported for the chlorination of phenols.
- selective chlorination
- phenols
- sulphuryl chloride
- sulphur-containing catalysts
- aromatic electrophilic substitution reactions
- monochlorination
- double chlorination
- para/ortho-isomer ratio
1. Commercial Applications of Chlorinated Phenols
As a class of compounds, chlorinated phenols are of substantial commercial interest. Already by 1936, 2,3,4,5,6-pentachlorophenol was being used as a wood preservative in the US; in addition, it was involved in the preparation of paints, adhesives, ropes, and insulation [1]. 2,4-Dichlorophenoxyacetic acid (2,4-D), manufacture of which involves 2,4-dichlorophenol, was first synthesized in 1941 and has been commercially produced in many parts of the world since the 1950s[2]. Subsequently, the range of applications of chlorinated phenols has expanded and they are currently useful both as synthetic intermediates for more complicated products and as end products themselves. The range of areas of application includes antiseptics, herbicides, pesticides, and dyes[3]. For example, 2,4-D and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) were the first herbicides produced commercially for weed control[4] and 2,4-D is still in widespread use in 2021[5]. In addition, 2,4,5-trichlorophenol acts as a leather and wood fungicide[6], 2,3,4,5,6-pentachlorophenol is used as an insecticide[7], 4-chloro-3,5-dimethylphenol is used as a household and hospital disinfectant, 4-chloro-2-methylphenol is used as a herbicide, and 4-chloro-3-methylphenol is used as an antiseptic and preservative [8][9][10]. Figure 1 shows some of the most common industrial products containing chlorinated phenolic components.
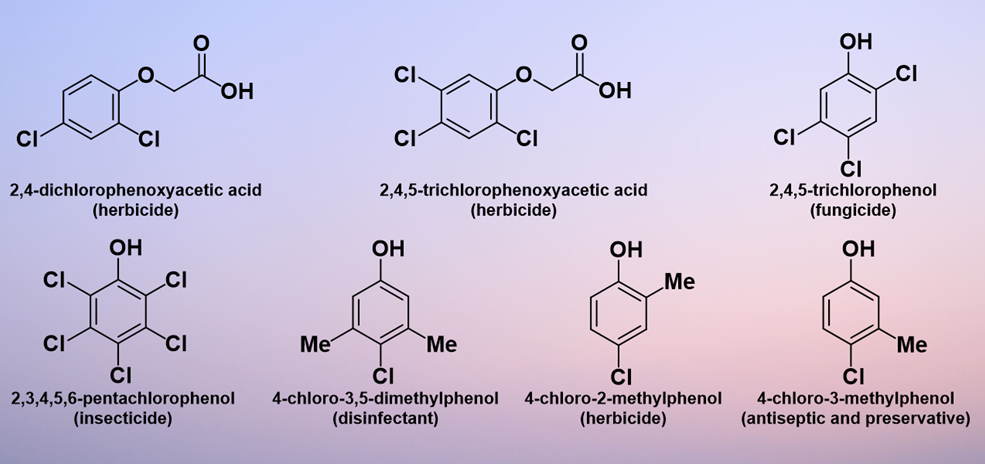

Figure 1. Common industrial products containing chlorinated phenol components.
2. Synthesis of Chlorinated Phenols
Chlorinated phenols are almost always manufactured by direct chlorination of the parent phenols. Many approaches have been attempted for such chlorinations[12][13][14][15][17][16][18][19][20][21][22][23], including use of a range of different chlorinating agents, but either chlorine gas or sulfuryl chloride is usually used in commercial processes. Chlorination using chlorine is not very regioselective for many phenols, and several important products require a pure para-chloro derivative. A review in 2021 [24] has reported on the development of various sulfur-containing catalysts and in particular poly(alkylene sulfide)s that render chlorination reactions of several simple phenols highly selective with sulfuryl chloride as the reagent[25][26][27][28][29][30][31][32][33][34].
This entry is adapted from the peer-reviewed paper 10.3390/org2030012
References
- Badanthadka, M.; Mehendale, H.M. Chlorophenols. Reference Module in Biomedical Sciences. Encyclopedia of Toxicology; 3rd ed.; pp. 896–899, 2014, doi:10.1016/B978-0-12-386454-3.00281-5.
- Garabrant, D.H.; Philbert, M.A. Review of 2,4-dichlorophenoxyacetic acid (2,4-D) epidemiology and toxicology. Crit. Rev. Toxicol. 2002, 32, 233–257, doi:10.1080/20024091064237.
- Ullmann’s Encyclopedia of Industrial Chemistry; 6th ed.; Wiley-VCH: Weinheim, Germany, 1998.
- Fagan, K.; Pollak J.K. The effect of the phenoxyacetic acid herbicides 2,4,5-trichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid as ascertained by direct experimentation. Residue Rev. 1984, 92, 29–58, doi:10.1007/978-1-4612-5266-5_2.
- EPA United State Environmental Protection Agency. Ingredients Used in Pesticide Products. 2,4-D. https://www.epa.gov/ingredients-used-pesticide-products/24-d (accessed on 01 August 2021).
- Favaro, G.; De Leo, D.; Pastore, P.; Magno, F.; Ballardin, A. Quantitative determination of chlorophenols in leather by pressurized liquid extraction and liquid chromatography with diode-array detection. J. Chromatogr. A 2008, 1177, 36–42, doi:10.1016/j.chroma.2007.10.106.
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: the public health perspective. Chemosphere 2011, 83, 1297–1306, doi:10.1155/2013/460215.
- World Health Organization, Stuart, M.C.; Kouimtzi, M.; Hill, S.R. ed.; WHO Model Formulary 2008.World Health Organization, 2009.
- Griffiths, C.; Barker, J.; Bleiker, T.O.; Chalmers, R.; Creamer, D. Rook’s Textbook of Dermatology; 9th ed.; John Wiley & Sons: Oxford, UK, 2017.
- Digison MB. A review of anti-septic agents for pre-operative skin preparation. Plast. Surg. Nurs. 2007, 27, 185–189, doi:10.1097/01.PSN.0000306182.50071.e2.
- Sullivan, J.D. para-Halogenation of phenols. US Pat. US 2,777,002, 1957. Chem. Abstr. 1957, 51, 77109.
- Nishihara, A.; Kato, H. Chlorination of phenols. Jpn. Pat. JP4,9035,344A, 1974. Chem. Abstr. 1974, 81, 120196.
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 4th ed.; Wiley: New York, USA, 1992.
- Xin, H.; Yang, S.; An, B.; An, Z. Selective water-based oxychlorination of phenol with hydrogen peroxide catalysed by manganous sulfate. RSC Adv. 2017, 7, 13467–13472, doi:10.1039/C7RA00319F.
- Nahide, P.D.; Ramadoss, V.; Juárez-Ornelas, K.A.; Satkar, Y.; Ortiz‐Alvarado, R.; Cervera‐Villanueva, J.M.J.; Alonso‐Castro, Á.J.; Zapata‐Morales, J.R.; Ramírez‐Morales, M.A.; Ruiz‐Padilla, A.J.; Deveze‐Álvarez, M.A.; Solorio‐Alvarado, C.R. In situ formed IIII -based reagent for the electrophilic ortho-chlorination of phenols and phenol ethers: The use of PIFA-AlCl3 System. Eur. J. Org. Chem. 2018, 2018, 485–493, doi:10.1002/ejoc.201701399.
- Xiong, X.; Yeung, Y-Y. Ammonium salt-catalyzed highly practical ortho-selective monohalogenation and phenylselenation of phenols: Scope and applications. ACS Catal. 2018, 8, 4033–4043, doi:10.1021/acscatal.8b00327.
- Bugnet, E.A.; Brough, A.R.; Greatrex, R.; Kee, T.P. On the para-selective chlorination of ortho-cresol. Tetrahedron 2002, 58, 8059–8065, doi:10.1016/S0040-4020(02)00996-1.
- Watson, W.D. Chlorination with sulfuryl chloride. US Pat. US 3,920,757A, 1975. Chem. Abstr. 1976, 84, 43612.
- Watson, W.D. The regioselective para chlorination of 2-methylphenol. Tetrahedron Lett. 1976, 17, 2591–2594, doi:10.1016/S0040-4039(00)91742-8.
- Watson, W.D. Regioselective para-chlorination of activated aromatic compounds. J. Org. Chem. 1985, 50, 2145–2148, doi:10.1021/jo00212a029.
- Binns, J.S.; Braithwaite, M.J. p-Chlorophenol. Ger. Offen. DE2,726,436A1, 1977. Chem. Abstr. 1978, 88, 120784.
- Ogata, Y.; Kimura, M.; Kondo, Y.; Katoh, H.; Chen, F.-C. Orientation in the chlorination of phenol and of anisole with sodium and t-butyl hypochlorites in various solvents. J. Chem. Soc. Perkin. Trans. 2 1984, 451–453, doi:10.1039/P29840000451.
- Olah, G.A.; Ohannesian, L.; Arvanaghi, M. Synthetic methods and reactions; 127. Regioselective para halogenation of phenols, phenol ethers and anilines with halodimethylsulfonium halides. Synthesis 1986, 868–870, doi:10.1055/s-1986-31813.
- Smith, K.; El-Hiti, G.A. Development of efficient and selective processes for the synthesis of commercially important chlorinated phenols. Organics 2021, 2, 142–160, doi:10.3390/org2030012.
- Tzimas, M.; Smith, K.; Brown, C.M.; Payne, K. Chlorination of aromatic compounds and catalysts therefor. Eur. Pat. EP0866049A2, 1998. Chem. Abstr. 1998, 129, 260219.
- Smith, K.; Tzimas, M.; Brown, C.M.; Payne, K. Dialkyl sulfides as selective catalysts for the chlorination of phenols. Sulfur Lett. 1999, 22, 89–101.
- Smith, K.; Williams, D.; El-Hiti, G.A. Regioselective chlorination of phenols in the presence of tetrahydrothiopyran derivatives. J. Sulfur Chem. 2019, 40, 529–538, doi:10.1080/17415993.2019.1620230.
- Tzimas, M.; Smith, K.; Brown, C.M.; Payne, K. Chlorination of aromatic compounds and catalysts therefor. Eur. Pat. EP0866048A2, 1998. Chem. Abstr. 1998, 129, 275696.
- Smith, K.; Tzimas, M.; Brown, C.M.; Payne, K. Dithiaalkanes and modified Merrifield resins as selective catalysts for the chlorination of phenols. Sulfur Lett. 1999, 22, 103–123.
- Smith, K.; Al-Zuhairi, A.J.; Elliot, M.C.; El-Hiti, G.A. Regioselective synthesis of important chlorophenols in the presence of methylthioalkanes with remote SMe, OMe or OH substituents. J. Sulfur Chem. 2018, 39, 607–621, doi:10.1080/17415993.2018.1493111.
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85, doi:10.1080/17415993.2014.965170.
- Smith, K.; El-Hiti, G.A.; Al-Zuhairi, A.J. The synthesis of polymeric sulfides by reaction of dihaloalkanes with sodium sulfide. J. Sulfur Chem. 2011, 32, 521–531, doi:10.1080/17415993.2011.616589.
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. The use of polymeric sulfides as catalysts for the para-regioselective chlorination of phenol and 2-chlorophenol. J. Sulfur Chem. 2020, 41, 1–12, doi:10.1080/17415993.2019.1688815.
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. para-Selective chlorination of cresols and m-xylenol using sulfuryl chloride in the presence of poly(alkylene sulfide)s. J. Sulfur Chem. 2020, 41, 345–356, doi:10.1080/17415993.2020.1740226.
