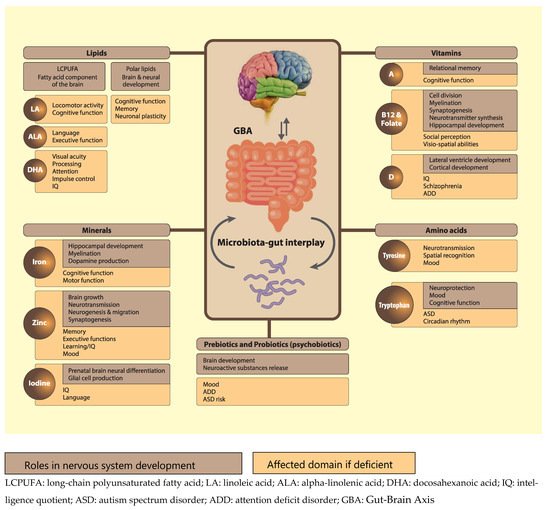
Proper nutrition is crucial for normal brain and neurocognitive development. Failure to optimise neurodevelopment early in life can have profound long-term implications for both mental health and quality of life. Although the first 1000 days of life represent the most critical period of neurodevelopment, the central and peripheral nervous systems continue to develop and change throughout life. Besides their individual contributions, the interaction of nutrients with other micro- and macronutrients and the way in which they are organised in the food matrix are all of crucial importance for normal neurocognitive development. Also the gut-brain axis, including the gut microbiota, is an important modifier in this respect.
- brain
- neurodevelopment
- childhood
- protein quality
- tyrosine
- tryptophan
- poly-unsaturated fatty acids
- polar lipids
- minerals
- vitamins
- kynurenine
- gut-brain axis
- prebiotics
- probiotics
1. Introduction
Nutrition is critical in supporting healthy brain development early in life, with long-lasting, and often, irreversible effects on an individual’s cognitive development and life-long mental health. In this review, we present recent human and pre-clinical evidence on the role of nutrition, with particular focus on more emerging nutrients, in neurocognitive development in healthy infants and children aged 0–59 months.
Development of the human brain starts with the closure of the neural tube by the fourth week of pregnancy [1] and the proliferation of neurons in the germinal layers near the ventricles during the early phases of gestation (from week six of pregnancy) [2]. This is followed by the migration of neurons to their final destination and simultaneous initiation of neuronal differentiation (Figure 1), adapted from [3]). Neuronal differentiation includes the formation of dendrites and axons, the production of neurotransmitters, the development of synapses and intracellular signaling systems, and establishment of complex neural membranes starting from late pregnancy until the first few months postnatally. The formation of synapses continues throughout life [2][4], whereas the production of various neurotransmitters starts prenatally and reaches mature levels around the age of three years [5]. In parallel, glial cell production begins during the second trimester (32 weeks of gestation) [2][5]; glial cells insulate the axons by surrounding them with a membranous myelin sheath (axonal myelination), a process that predominantly takes place between the second trimester of gestation and the first year of life. Myelination continues, but starts to decline in early adulthood after which it stops at around the age of 40 years [2].
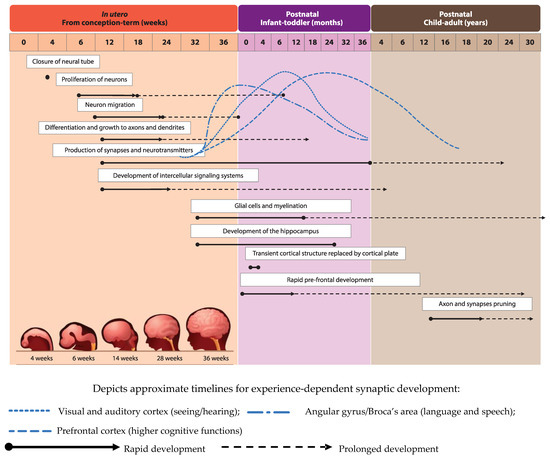
Development of brain structures occurs in several phases. The major transition takes place when the transient cortical structure, mediating fetal and neonatal behavior, is replaced by the cortical plate at three to four months of age. As a result, motor behavior changes from non-directed general movements to more goal-directed movements, such as reaching. The hippocampus, which is important for facial and scene-recognition, as well as spatial memory, develops at approximately 32 weeks of gestation until at least 18 months postnatally [4][5]. The prefrontal cortex, responsible for complex processing tasks such as attention and multi-tasking, exhibits initial rapid development during the first 6 months of life [5]. Note, that prefrontal development continues well into the third decade of life [6]. The pruning of axons and synapses to further optimize the brain’s functioning usually starts between the onset of puberty and early adulthood [2].
2. Nutrients that Play a Role in Neurodevelopment
2.1. Lipids
2.1.1. Long-Chain Polyunsaturated Fatty Acids
Neurodevelopment is influenced by a number of factors ranging from gestational age at birth and social environment to nutrition. Dietary fat in particular is an important modifiable nutritional factor illustrated by the crucial role of the polyunsaturated fatty acids (PUFAs) linoleic acid (LA; 18:2 n-6), alpha-linoleic acid (ALA, 18:3 n-3), docosahexanoic acid (DHA; 22:6 n-3) and arachidonic acid (AA, 20:4 n-6) in normal brain formation and neuronal myelination during infant neurodevelopment [2][7]. Furthermore, long-chain PUFAs (LCPUFAs) have been shown to affect the production of various neurotransmitters [5], with profound effects on monoaminergic, cholinergic, and gamma-aminobutyric acid (GABA) ergic systems. DHA is especially important for visual and prefrontal cortex development, the latter of which mediates attention, inhibition and impulsivity actions [5].
In addition to sufficient intake via diet or supplementation, a balanced ratio between LA and ALA is important as well [7]. In the prospective Mothers and Children’s Environmental Health (MOCEH) cohort of 960 pregnant women in Korea, an inverse association between LA/ALA ratio during pregnancy and Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) scores in the offspring at six months of age was reported [8]. The average LA/ALA ratio in this population amounted to 11.12 ± 6.9.
LA is abundantly present in daily food and high intake can have negative health consequences [7]. High LA in colostrum and breastmilk has been associated with poorer motor and cognitive scores at two and three years of age [9] and a lower verbal intelligence quotient (IQ) at five-to-six years [10] in the observational EDEN (Etude de cohorte généraliste, menée en France sur les Déterminants pré et post natals précoces du développement psychomoteur et de la santé de l’Enfant) cohort in France. This negative impact was postulated to take effect through several mechanisms, namely the suppression of biosynthesis of n-3 PUFAs (due to enzymatic competition to convert n-6 and n-3 PUFA to LCPUFA), which supplies necessary DHA for brain development, as well as by decreased uptake of circulating DHA by the brain and thus impaired accretion of DHA in the brain. Lastly, n-6 PUFAs are precursors for several pro-inflammatory eicosanoids that can be produced in early life and may have a negative impact on cognitive function [10].
Several observational studies reported that high DHA levels in pre- and postnatal periods seem to improve specific cognitive skills ranging from processing ability and attention to overall IQ in the offspring, even up to 12 years of life [11][12][13]. Nonetheless, the impact of DHA supplementation during pregnancy remains controversial. Daily 400 mg DHA supplementation for 20 weeks in pregnant mothers showed positive effects on the infant’s attention ability at 5 years of age [14], while an earlier study among 2499 pregnant women in Australia found that daily 800 mg DHA supplementation did not affect cognitive and language development of the offspring at 12 and 18 months [15]. The latter is further supported by the results of a Cochrane systematic review (2015) stating that there was no effect of DHA supplementation in breastfeeding mothers on language development, problem-solving abilities, psychomotor development, and general movement ability of their offspring [16]. Another Cochrane review (2017) reported that, although there was no concern over the safety of infant formulas supplemented with DHA and AA, the majority of evaluated randomized controlled trials (RCTs) did not show any beneficial effects on neurodevelopmental outcomes in term infants [17]. In addition, the authors concluded that the positive effect on visual acuity had not been consistently demonstrated. Although the review included the study by Colombo et al. [18], reporting a beneficial effect of DHA/AA supplementation (up to 0.64% DHA/0.64% AA) in infant formula on problem-solving skills at nine months [18], it did not include earlier studies on the topic [19][20]. These studies indicated that routine supplementation of term infant milk formula with DHA (at the level of 0.3% PUFA), from birth to four months of age, was associated with improved neurodevelopmental outcome at four months [20] and higher MDI scores at 18 months [19]. In addition, better inhibitory control measured by behavioral and brain electrophysiology responses among those supplemented with the above-mentioned dose at 5.5 years of age has been described as well [21]. Importantly, dosing LCPUFAs at a level higher than 0.64% in early life may have negative effects on cognitive development at a later stage [18].
The impact of essential fatty acids (EFA) and LCPUFAs on cognition and brain development appears to be particularly evident in older children. Interestingly, DHA and AA supplementation of 200 mg daily in Growing Up Milk (GUM) among toddlers aged 13 months for the duration of one year increased the Bayley III language composite score at 24 months as compared to those receiving standard GUM without LCPUFA. The same study reported fewer inattention episodes among boys receiving LCPUFA-supplemented GUM as compared to their unsupplemented counterparts [22].
Two separate studies in two-to-six years old children in Ghana and Tanzania revealed that children with the highest levels of blood EFA and DHA had at least a three times higher chance of successfully passing an executive function test [23][24]. In older pre-school children, the consumption of 978 g of fish over one week influenced cognitive function compared to those consuming 850 mg of meat, after adjusting for dietary compliance; information on EFA and DHA blood levels was not included [25].
2.1.2. Polar Lipids
Polar lipids are amphiphilic in nature and contain a hydrophobic tail and a hydrophilic head. Phospholipids (glycerophospholipids and sphingomyelin) and sphingolipids (ceramides, cerebrosides and gangliosides) are the main representatives of this group. Polar lipids make up biological membranes but are also found in circulating fluids. In mammalian milk, the milk fat globule membrane (MFGM), the trilayer membrane structure surrounding each fat globule, is an important source of polar lipids [26][27], as are nanovesicles (exosomes). Nanovesicles are secreted into milk by mammary gland cells and are implicated in cell-to-cell communication by virtue of their functionally active cargo (e.g., messenger-RNA (mRNA), micro-RNA (miRNA), and different proteins; [28]). Human as well as bovine milk contains approximately 4% fat in the form of fat globules [29]. These globules are filled with triglycerides, constituting 98% of total fat. The lipids within the MFGM primarily include polar lipids but also comprise neutral lipids (like cholesterol). The main polar lipids present in human and bovine MFGMs are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and sphingomyelin (SM) [30][31]; human milk contains higher levels of SM and PS, whereas relatively more PE is present in bovine milk fat [32].
To date, no intake recommendations or guidelines for polar lipids have been proposed or implemented by health authorities. However, adequate intakes have been defined for two nutrients that serve as structural parts of polar lipids, choline and DHA [33][34]. Presently, only limited scientific evidence exists on the brain bioavailability of polar lipids via placental transfer or transport over the blood brain barrier (BBB) [35][36][37]. Nevertheless, supplementing polar lipids in wild-type animal models and healthy infants does suggest benefits for cognitive performance.
The putative role of MFGM polar lipids in brain and neurocognitive development has received significant attention. In Sprague-Dawley rats, oral gavage supplementation with MFGM led to neurocognitive benefits by early upregulation of genes involved in brain function, such as brain-derived neurotrophic factor (BDNF) and St8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 [38]. Somewhat unexpectedly, a human RCT evaluating the effects of maternal dietary supplementation of complex milk lipids (CML; gangliosides and phospholipids) from the MFGM during pregnancy on fetal growth showed no effects on any of the fetal biometric dimensions measured [39]. The lack of effect could be due to the application of an inadequate dose of polar lipids. In a Belgian study, a phospholipid-rich MFGM concentrate given daily to preschool children aged 2.5–6 years for a period of four months decreased behavioral problems and reduced days with fever during the intervention period [40]. Notwithstanding this encouraging result, Timby and colleagues (2017) concluded that while MFGM interventions seem safe, it is still unclear which MFGM fractions are most suitable for supplementation and at what concentration at which age. Furthermore, it was pointed out that the evidence base for the effects of MFGM polar lipids on brain and neurocognitive development is still limited [41]. Since then, several studies have provided additional evidence for the importance of the MFGM in early life with mixed outcomes. A study in 451 healthy term infants showed that receiving formula with added bovine MFGM and bovine lactoferrin (LF) resulted in accelerated neurodevelopment at day 365 as evidenced by higher mean cognitive (+8.7), language (+12.3), and motor (+12.6) Bayley-III scores, and improved global development scores from day 120 to day 275 and attention at day 365 in the MFGM + LF group [42]. In addition, enhanced language skills at day 545 were observed (some subcategories of the MacArthur-Bates Communicative Development Inventories were higher in the MFGM + LF group).
There has been growing interest in the use of gangliosides as part of a supplement for either the infant or the mother because these polar lipids serve a crucial role in pre- and postnatal development of the brain, which coincides with the critical window of rapid brain growth around birth [43]. Ceramides are essential for neural development contributing to ganglioside synthesis in utero. Interestingly, the phlorizin domain of the lactase enzyme splits ceramides from glucosyl, galactosyl, and lactosyl-cerebrosides [44]. In addition, lactase splits lactose into glucose and galactose. As nearly all healthy infants are lactase persistent, lactase activity in their small intestine yields ceramide, glucose, and galactose moieties from dietary lactose and glycosphingolipid intake, all important building blocks for the developing nervous system. Gangliosides are glycolipids that contain sialic acid, which is an essential nutrient for optimal brain development and cognition. Endogenous production of sialic acid is possible but limited. Rather, it is available in human milk oligosaccharides in relatively large quantities, predominantly in the form of Neu5Ac (N-acetylneuraminic acid), which is the precursor of various neural brain glycoproteins, including polysialic acid, gangliosides, glycosaminoglycans, and mucins. The major protein carrier of polysialic acids is NCAM (Neural Cell Adhesion Molecule); polysialylated-NCAM is a key neuroplastic molecule involved in neuronal plasticity and of crucial importance for memory formation. Other examples of sialylated proteins are synaptic cell adhesion molecule 1 and scavenger receptor CD36 [45]. Sialic acid is particularly abundant in neuronal cell membranes. Notably, the location and amount of sialic acid in different regions of the brain change dramatically during development.
The high sialic acid content of human breast milk in addition to its role in brain development suggest that sialic acid in breast milk has an impact on infant cognitive development. This could also imply that brain growth creates a greater need for sialic acid than can be provided by endogenous biosynthesis in the infant. This is supported by findings from a study in which sialic acid was measured in brain samples from infants (1–38 weeks) that died of sudden infant death showing that the sialic acid content was higher in the brains of breast-fed infants than in those of formula-fed infants [46].
2.2. Minerals
2.2.1. Iron
In addition to lipids, micronutrients are critical for normal neurodevelopment as well. Iron, for instance, is vital for energy production, oxygen transportation, and DNA synthesis. It plays a crucial role in hippocampal development, myelination and production of neurotransmitters, such as dopamine, serotonin, and norepinephrine as shown in pre-clinical studies [5][47][48]. Iron deficiency results in reduced 6-desaturase enzyme activity that is required for the synthesis of essential fatty acids and can therefore impair the synthesis of α
-linoleic acid (ALA, 18:3 n-3) into DHA [49]. Currently, no literature is available on the impact of combined iron and LCPUFA deficiencies on cognitive neurodevelopment.
Limited evidence from recent studies suggest that iron supplementation during pregnancy and infancy may positively influence the psychomotor development of children [47][48][50]. A positive effect of prenatal iron supplementation during pregnancy on overall cognitive development of the child has been described only for anemic pregnant women. In this group, a favorable effect on cognitive performance in children under two years of age, toddlers and primary school children was observed [48][50][51].
Based on a systematic review and a follow-up study, the effects of iron supplementation during early life on cognitive function are unclear at 12 months of age. In addition, no benefit on cognitive function at 18 months could be detected [47][50]. Notably, the provision of iron to iron-replete infants could have a negative effect on long-term cognitive development as shown in a cohort study among infants in Chile [47][52]. Positive impact of iron supplementation on cognitive function seems to be observed only in anemic primary school children [47][51]. Based on the available evidence, adequate dietary iron intake should be encouraged during pregnancy and post-natal life up to adulthood. With regard to iron supplementation, a different picture emerges. Given the uncertainty of the efficacy of iron supplementation due to significant supplementation heterogeneity across the various studies (i.e., type and format of iron supplementation, dosage, length of supplementation, presence of other nutrients) [47][50], combined with the potential negative impact of providing high iron dosages to iron-replete infants [52], it seems advisable to restrict the provision of iron supplements, in the right form and dosage, to anemic individuals.
2.2.2. Zinc
Zinc is necessary for central nervous system (CNS) development and is one of the most ubiquitous metals found in the brain; it is present in many enzymes involved in brain growth and is important for neurotransmission. In animal models, zinc has been shown to be involved in neurogenesis, neural migration, synaptogenesis, and regulation of GABA-Ergic neurotransmitter release [5][53].
Zinc deficiency during pregnancy and early infancy has long been associated with developmental deficits, such as poorer learning, attention, memory, and mood [5]. However, there is currently no convincing evidence that maternal zinc supplementation improves cognitive development in the offspring [54][55].
Evidence on the association between zinc intake and cognitive development is quite limited for infants. Six-month old infants receiving either a combination of micronutrients (10 mg/d zinc, 10 mg/d iron and 0.5 mg/d copper) or iron and copper alone for the duration of 6 months presented with different outcomes. In the group receiving zinc, there was an improvement in normative information processing and active attentional profiles at two years of age. However, no differences were reported regarding other parameters, which included Bayley Scales of Infant Development (BSID) at six, 12, and 18 months. Infants receiving zinc supplementation were also able to maintain a better zinc status [55].
A Cochrane systematic review (2012) concluded that there is no significant effect of zinc intake on mental and motor development in children [56]. This was nuanced by another systematic review, published later that year, stating that the effect of zinc supplementation on cognitive function might be dependent on the dose of supplementation and the duration of the intervention [57].
2.2.3. Iodine
Iodine plays an important role in brain development in the form of thyroxine and triiodothyronine. It affects the timing of differentiation of neural tissue in the brain prenatally and determines the number of glial cells for myelin sheath production postnatally. Maternal thyroid hormone can be found in the embryonic cavities at the end of week 4 post-conception when the formation of the brain cortex and the anterior part of the neural tube takes place [58].
The impact of pre-conception iodine levels has been recently investigated in the Southampton Women Cohort [59]. The results revealed that a low maternal urinary iodine concentration, measured by iodine/creatinine (I/Cr) ratio, at 3.3 years before conception was associated with low overall childhood cognitive function at 6–7 years as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI) [59]. Around 8.9% of the women in this cohort presented with a low I/Cr ratio. Unexpectedly, the same study reported no influence of pre-conception iodine levels on specific measures of executive function at the age of six-to-seven years [59].
The Generation R cohort study showed that mild-to-moderate iodine deficiency in early pregnancy affects the offspring’s behavior and risk for development of ADHD at eight years of age [60]. Severe iodine deficiency during pregnancy is well-known to result in maternal and fetal hypothyroidism and has been shown to be associated with serious adverse health effects in the offspring, including congenital hypothyroidism, growth retardation and impaired cognition encompassing deficits in hearing, speech, gait and IQ [5][61]. Still, several iodine supplementation studies during pregnancy on offspring cognitive function reported inconclusive findings. This, in part, may be explained by the application of age-inappropriate global development assessments that may have caused misclassification and lack of correlation with cognitive function at that particular time [58][62][63].
Post-natally, iodine continues to play a role in neurocognitive development. The level of iodine in colostrum predicts the motor development capability of infants at 18 months, but does not relate to other abilities, such as language development or overall cognition [64]. Interestingly, a study on iodine supplementation using iodized salts for children in areas where the incidence of iodine deficiency is high, reported no benefits on cognitive function in children older than three years of age despite the improvement in iodine status [65].
2.3. Vitamins
Despite extensive research conducted on vitamin supplementation, only limited recent evidence exists to suggest that vitamin supplementation during pregnancy and early intake by infants positively influences cognitive development of children. Nevertheless, vitamin A, vitamin B12 (cobalamin), folate, and vitamin D are well-recognized for their capacity to critically influence early cognitive development [5][49], and micronutrient deficiencies early in life can lead to impairments of the CNS.
2.3.1. Vitamin A
2.3.2. Vitamin B12
2.3.3. Vitamin D
2.4. Dietary Protein and Amino Acids
A classic example of the importance of proteins to behavioral and neurocognitive development in infants is the longitudinal study by Chavez et al. [79] which evaluated the effects of nutritional supplementation on infants’ physical, mental, and social development in two groups of 17 mother-child pairs in a poor rural Mexican community. In this study, one group of mothers was supplemented daily with 205 calories and 15 g of protein during pregnancy and 305 calories and 15 g of protein during lactation, whilst the other group followed the usual feeding habits of the community. Between the 12th and 16th week of life, the supplemented infants began to receive whole cow’s milk ad libitum and prepared baby food in quantities sufficient to maintain adequate rates of growth. At 18 months of intervention, the mothers of supplemented children displayed more complex interactions with their children, who were more restless, playful, demanding and disobedient than those non-supplemented. These results suggest a beneficial effect of protein (and energy) supplementation on the behavioral patterns within the family, with the more active children eliciting greater stimulation from their parents. Another historic study [80] demonstrated that protein supplementation, rather than energy, during early childhood improved psycho-educational performance. Therefore, Guatemalan children exposed to protein supplement scored significantly higher on tests of knowledge, numerical aptitude, reading and vocabulary as compared to those that only received energy supplementation. Two decades later, 130 female subjects were re-evaluated and, interestingly, women exposed to protein supplementation during early childhood had better educational achievements than those from the energy group [81].
2.4.1. The Importance of Protein Quality
2.4.2. mTORC1: Linking Protein (in)Adequacy to Brain Development
3. Nutrient Interactions through the Gut-Brain Axis (GBA)
3.1. What Is the Microbiome Gut-Brain Axis?
3.3. The Impact of Pro- and Prebiotics through the GBA
This entry is adapted from the peer-reviewed paper 10.3390/nu13010199
References
- Black, M.M. Impact of nutrition on growth, brain, and cognition. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 185–195.
- Hadders-Algra, M. Effect of long-chain polyunsaturated fatty acid supplementation on neurodevelopmental outcome in full-term infants. Nutrients 2010, 2, 790–804.
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B.; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70.
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321.
- Cusick, S.E.; Georgieff, M.K. The role of nutrition in brain development: The golden opportunity of the “first 1000 days”. J. Pediatr. 2016, 175, 16–21.
- Tamnes, C.K.; Herting, M.M.; Goddings, A.L.; Meuwese, R.; Blakemore, S.J.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.A.; et al. Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017, 37, 3402–3412.
- Kim, H.; Lee, E.; Kim, Y.; Ha, E.H.; Chang, N. Association between maternal intake of n-6 to n-3 fatty acid ratio during pregnancy and infant neurodevelopment at 6 months of age: Results of the MOCEH cohort study. Nutr. J. 2017, 16, 23.
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17.
- Bernard, J.Y.; Armand, M.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr. Res. 2015, 77, 829–835.
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5–6 years. J. Pediatr. 2017, 183, 43–50.e3.
- Dalmeijer, G.W.; Wijga, A.H.; Gehring, U.; Renders, C.M.; Koppelman, G.H.; Smit, H.A.; van Rossem, L. Fatty acid composition in breastfeeding and school performance in children aged 12 years. Eur. J. Nutr. 2016, 55, 2199–2207.
- Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Frøyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA status during pregnancy has a positive impact on infant problem solving: A Norwegian prospective observation study. Nutrients 2018, 10, 529.
- Rees, A.; Sirois, S.; Wearden, A. Prenatal maternal docosahexaenoic acid intake and infant information processing at 4.5mo and 9mo: A longitudinal study. PLoS ONE 2019, 14, e0210984.
- Ramakrishnan, U.; Gonzalez-Casanova, I.; Schnaas, L.; DiGirolamo, A.; Quezada, A.D.; Pallo, B.C.; Hao, W.; Neufeld, L.M.; Rivera, J.A.; Stein, A.D.; et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1075–1082.
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; DOMInO, I.T. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683.
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015.
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376.
- Colombo, J.; Jill Shaddy, D.; Kerling, E.H.; Gustafson, K.M.; Carlson, S.E. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 52–56.
- Drover, J.R.; Hoffman, D.R.; Castañeda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230.
- Koletzko, B.; Uauy, R.; Palou, A.; Kok, F.; Hornstra, G.; Eilander, A.; Moretti, D.; Osendarp, S.; Zock, P.; Innis, S. Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in children—A workshop report. Br. J. Nutr. 2010, 103, 923–928.
- Liao, K.; McCandliss, B.D.; Carlson, S.E.; Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Lepping, R.J.; Sittiprapaporn, W.; Cheatham, C.L.; Gustafson, K.M. Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev. Sci. 2017, 20, e12455.
- Devlin, A.M.; Chau, C.M.Y.; Dyer, R.; Matheson, J.; McCarthy, D.; Yurko-Mauro, K.; Innis, S.M.; Grunau, R.E. Developmental outcomes at 24 months of age in toddlers supplemented with arachidonic acid and docosahexaenoic acid: Results of a double blind randomized, controlled trial. Nutrients 2017, 9, 975.
- Jumbe, T.; Comstock, S.S.; Harris, W.S.; Kinabo, J.; Pontifex, M.B.; Fenton, J.I. Whole-blood fatty acids are associated with executive function in Tanzanian children aged 4-6 years: A cross-sectional study. Br. J. Nutr. 2016, 116, 1537–1545.
- Adjepong, M.; Yakah, W.; Harris, W.S.; Annan, R.A.; Pontifex, M.B.; Fenton, J.I. Whole blood n-3 fatty acids are associated with executive function in 2-6-year-old Northern Ghanaian children. J. Nutr. Biochem. 2018, 57, 287–293.
- Øyen, J.; Kvestad, I.; Midtbø, L.K.; Graff, I.E.; Hysing, M.; Stormark, K.M.; Markhus, M.W.; Baste, V.; Frøyland, L.; Koletzko, B.; et al. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018, 16, 41.
- Gallier, S.; Gragson, D.; Cabral, C.; Jiménez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511.
- Lopez, C.; Madec, M.-N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. 2010, 120, 22–33.
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J. Nutr. 2017, 147, 3–10.
- Hageman, J.H.J.; Danielsen, M.; Nieuwenhuizen, A.G.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49.
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457.
- Zou, X.; Guo, Z.; Jin, Q.; Huang, J.; Cheong, L.; Xu, X.; Wang, X. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chem. 2015, 185, 362–370.
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016, 14, e04484.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461.
- Zheng, L.; Fleith, M.; Giuffrida, F.; O’Neill, B.V.; Schneider, N. Dietary polar lipids and cognitive development: A narrative review. Adv. Nutr. 2019, 10, 1163–1176.
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk polar lipids: Underappreciated lipids with emerging health benefits. Nutrients 2020, 12, 1001.
- Schipper, L.; van Dijk, G.J.; van der Beek, E.M. Milk lipid composition and structure; the relevance for infant brain development. OCL 2020, 27, 5.
- Brink, L.R.; Lönnerdal, B. The role of milk fat globule membranes in behavior and cognitive function using a suckling rat pup supplementation model. J. Nutr. Biochem. 2018, 58, 131–137.
- Norris, T.; Souza, R.; Xia, Y.; Zhang, T.; Rowan, A.; Gallier, S.; Zhang, H.; Qi, H.; Baker, P. Effect of supplementation of complex milk lipids in pregnancy on fetal growth: Results from the Complex Lipids in Mothers and Babies (CLIMB) randomized controlled trial. J. Matern. Fetal Neonatal Med. 2019, 1–10.
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752.
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Supplementation of infant formula with bovine milk fat globule membranes. Adv. Nutr. 2017, 8, 351–355.
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. J. Pediatr. 2019, 215, 24–31.
- Ryan, J.M.; Rice, G.E.; Mitchell, M.D. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887.
- Nilsson, Å. Role of sphingolipids in infant gut health and immunity. J. Pediatr. 2016, 173, S53–S59.
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S.
- Röhrig, C.H.; Choi, S.S.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038.
- Larson, L.M.; Phiri, K.S.; Pasricha, S.R. Iron and cognitive development: What is the evidence? Ann. Nutr. Metab. 2017, 71 (Suppl. 3), 25–38.
- Chmielewska, A.; Dziechciarz, P.; Gieruszczak-Białek, D.; Horvath, A.; Pieścik-Lech, M.; Ruszczyński, M.; Skórka, A.; Szajewska, H. Effects of prenatal and/or postnatal supplementation with iron, PUFA or folic acid on neurodevelopment: Update. Br. J. Nutr. 2019, 122, S10–S15.
- González, H.F.; Visentin, S. Micronutrients and neurodevelopment: An update. Arch. Argent Pediatr. 2016, 114, 570–575.
- Szajewska, H.; Ruszczynski, M.; Chmielewska, A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2010, 91, 1684–1690.
- Low, M.S.; Speedy, J.; Styles, C.E.; De-Regil, L.M.; Pasricha, S.R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 4, CD009747.
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs. low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215.
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317.
- Caulfield, L.E.; Putnick, D.L.; Zavaleta, N.; Lazarte, F.; Albornoz, C.; Chen, P.; Dipietro, J.A.; Bornstein, M.H. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. Am. J. Clin. Nutr. 2010, 92, 130–136.
- Colombo, J.; Zavaleta, N.; Kannass, K.N.; Lazarte, F.; Albornoz, C.; Kapa, L.L.; Caulfield, L.E. Zinc supplementation sustained normative neurodevelopment in a randomized, controlled trial of Peruvian infants aged 6–18 months. J. Nutr. 2014, 144, 1298–1305.
- Gogia, S.; Sachdev, H.S. Zinc supplementation for mental and motor development in children. Cochrane Database Syst. Rev. 2012, 12, CD007991.
- Nissensohn, M.; Sánchez-Villegas, A.; Fuentes Lugo, D.; Henríquez Sánchez, P.; Doreste Alonso, J.; Skinner, A.L.; Medina, M.W.; Lowe, N.M.; Hall Moran, V.; Serra-Majem, L. Effect of zinc intake on mental and motor development in infants: A meta-analysis. Int J. Vitam. Nutr. Res. 2013, 83, 203–215.
- Bell, M.A.; Ross, A.P.; Goodman, G. Assessing infant cognitive development after prenatal iodine supplementation. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 928S–934S.
- Robinson, S.M.; Crozier, S.R.; Miles, E.A.; Gale, C.R.; Calder, P.C.; Cooper, C.; Inskip, H.M.; Godfrey, K.M. Preconception maternal iodine status is positively associated with iq but not with measures of executive function in childhood. J. Nutr. 2018, 148, 959–966.
- Modesto, T.; Tiemeier, H.; Peeters, R.P.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Ghassabian, A. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. 2015, 169, 838–845.
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 918S–923S.
- Zou, Y.; Ding, G.; Lou, X.; Mo, Z.; Zhu, W.; Mao, G.; Zhou, J. A study on the influencing factors of urinary iodine concentration and the relationship between iodised salt concentration and urinary iodine concentration. Br. J. Nutr. 2015, 113, 142–146.
- Murcia, M.; Espada, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.J.; Vioque, J.; Basterrechea, M.; Riaño, I.; González, L.; Alvarez-Pedrerol, M.; et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: The INMA Mother and Child Cohort Study. J. Epidemiol. Community Health 2018, 72, 216–222.
- Wu, M.; Wu, D.; Wu, W.; Li, H.; Cao, L.; Xu, J.; Yu, X.; Bian, X.; Yan, C.; Wang, W. Relationship between iodine concentration in maternal colostrum and neurobehavioral development of infants in Shanghai, China. J. Child Neurol. 2016, 31, 1108–1113.
- Aboud, F.E.; Bougma, K.; Lemma, T.; Marquis, G.S. Evaluation of the effects of iodized salt on the mental development of preschool-aged children: A cluster randomized trial in northern Ethiopia. Matern. Child Nutr. 2017, 13, e12322.
- Etchamendy, N.; Enderlin, V.; Marighetto, A.; Pallet, V.; Higueret, P.; Jaffard, R. Vitamin A deficiency and relational memory deficit in adult mice: Relationships with changes in brain retinoid signalling. Behav. Brain Res. 2003, 145, 37–49.
- Lima, A.A.; Kvalsund, M.P.; Souza, P.P.; Figueiredo, Í.L.; Soares, A.M.; Mota, R.M.; Lima, N.L.; Pinkerton, R.C.; Patrick, P.P.; Guerrant, R.L.; et al. Zinc, vitamin A, and glutamine supplementation in Brazilian shantytown children at risk for diarrhea results in sex-specific improvements in verbal learning. Clinics (Sao Paulo) 2013, 68, 351–358.
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335.
- Lai, J.S.; Cai, S.; Feng, L.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; Godfrey, K.M.; Meaney, M.J.; Rifkin-Graboi, A.; et al. Associations of maternal zinc and magnesium with offspring learning abilities and cognitive development at 4 years in GUSTO. Nutr. Neurosci. 2019, 1–10.
- Kvestad, I.; Hysing, M.; Shrestha, M.; Ulak, M.; Thorne-Lyman, A.L.; Henjum, S.; Ueland, P.M.; Midttun, Ø.; Fawzi, W.; Chandyo, R.K.; et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am. J. Clin. Nutr. 2017, 105, 1122–1131.
- Strand, T.A.; Taneja, S.; Ueland, P.M.; Refsum, H.; Bahl, R.; Schneede, J.; Sommerfelt, H.; Bhandari, N. Cobalamin and folate status predicts mental development scores in North Indian children 12–18 mo of age. Am. J. Clin. Nutr. 2013, 97, 310–317.
- Strand, T.A.; Ulak, M.; Chandyo, R.K.; Kvestad, I.; Hysing, M.; Shrestha, M.; Basnet, S.; Ranjitkar, S.; Shrestha, L.; Shrestha, P.S. The effect of vitamin B12 supplementation in Nepalese infants on growth and development: Study protocol for a randomized controlled trial. Trials 2017, 18, 187.
- Rauh-Pfeiffer, A.; Handel, U.; Demmelmair, H.; Peissner, W.; Niesser, M.; Moretti, D.; Martens, V.; Wiseman, S.; Weichert, J.; Heene, M.; et al. Three-month B vitamin supplementation in pre-school children affects folate status and homocysteine, but not cognitive performance. Eur. J. Nutr. 2014, 53, 1445–1456.
- O’Loan, J.; Eyles, D.W.; Kesby, J.; Ko, P.; McGrath, J.J.; Burne, T.H. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007, 32, 227–234.
- McGrath, J.; Eyles, D.; Mowry, B.; Yolken, R.; Buka, S. Low maternal vitamin D as a risk factor for schizophrenia: A pilot study using banked sera. Schizophr. Res. 2003, 63, 73–78.
- Whitehouse, A.J.; Holt, B.J.; Serralha, M.; Holt, P.G.; Hart, P.H.; Kusel, M.M. Maternal vitamin D levels and the autism phenotype among offspring. J. Autism Dev. Disord. 2013, 43, 1495–1504.
- Zhu, P.; Tong, S.L.; Hao, J.H.; Tao, R.X.; Huang, K.; Hu, W.B.; Zhou, Q.F.; Jiang, X.M.; Tao, F.B. Cord blood vitamin D and neurocognitive development are nonlinearly related in toddlers. J. Nutr. 2015, 145, 1232–1238.
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413.
- Chavez, A.; Martinez, C.; Yaschine, T. Nutrition, behavioral development, and mother-child interaction in young rural children. Fed. Proc. 1975, 34, 1574–1582.
- Pollitt, E.; Gorman, K.S.; Engle, P.L.; Martorell, R.; Rivera, T.; Wachs, T.D.; Scrimshaw, N.S. Early supplemenatry feeding and cognition: Effects over two decades. Monogr. Soc. Res. Child Dev. 1993, 58, 1–99.
- Li, H.; Barnhart, H.X.; Stein, A.D.; Martorell, R. Effects of early childhood supplementation on the educational achievement of women. Pediatrics 2003, 112, 1156–1162.
- Morgane, P.J.; Mokler, D.J.; Galler, J.R. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci. Biobehav. Rev. 2002, 26, 471–483.
- Chertoff, M. Protein malnutrition and brain development. Brain Disord. Ther. 2015, 4, 168.
- Laus, M.F.; Vales, L.D.; Costa, T.M.; Almeida, S.S. Early postnatal protein-calorie malnutrition and cognition: A review of human and animal studies. Int. J. Environ. Res. Public Health 2011, 8, 590–612.
- Hemb, M.; Cammarota, M.; Nunes, M.L. Effects of early malnutrition, isolation and seizures on memory and spatial learning in the developing rat. Int. J. Dev. Neurosci. 2010, 28, 303–307.
- Valadares, C.T.; Fukuda, M.T.; Françolin-Silva, A.L.; Hernandes, A.S.; Almeida, S.S. Effects of postnatal protein malnutrition on learning and memory procedures. Nutr. Neurosci. 2010, 13, 274–282.
- Zhang, Y.; Li, N.; Yang, Z. Perinatal food restriction impaired spatial learning and memory behavior and decreased the density of nitric oxide synthase neurons in the hippocampus of adult male rat offspring. Toxicol. Lett. 2010, 193, 167–172.
- Harris, T.E.; Lawrence, J.C. TOR signaling. Sci. STKE 2003, 2003, re15.
- Gal-Ben-Ari, S.; Kenney, J.W.; Ounalla-Saad, H.; Taha, E.; David, O.; Levitan, D.; Gildish, I.; Panja, D.; Pai, B.; Wibrand, K.; et al. Consolidation and translation regulation. Learn Mem. 2012, 19, 410–422.
- Burket, J.A.; Benson, A.D.; Tang, A.H.; Deutsch, S.I. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 60–65.
- Ishizuka, Y.; Kakiya, N.; Nawa, H.; Takei, N. Leucine induces phosphorylation and activation of p70S6K in cortical neurons via the system L amino acid transporter. J. Neurochem. 2008, 106, 934–942.
- Huang, Y.; Kang, B.N.; Tian, J.; Liu, Y.; Luo, H.R.; Hester, L.; Snyder, S.H. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J. Neurosci. 2007, 27, 449–458.
- Quevedo, C.; Salinas, M.; Alcázar, A. Regulation of cap-dependent translation by insulin-like growth factor-1 in neuronal cells. Biochem. Biophys. Res. Commun. 2002, 291, 560–566.
- Lee, C.C.; Huang, C.C.; Wu, M.Y.; Hsu, K.S. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J. Biol. Chem. 2005, 280, 18543–18550.
- Avruch, J.; Hara, K.; Lin, Y.; Liu, M.; Long, X.; Ortiz-Vega, S.; Yonezawa, K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 2006, 25, 6361–6372.
- Chenal, J.; Pierre, K.; Pellerin, L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur. J. Neurosci. 2008, 27, 53–65.
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317.
- Nawa, H.; Takei, N. BDNF as an anterophin; a novel neurotrophic relationship between brain neurons. Trends Neurosci. 2001, 24, 683–684.
- Takei, N.; Kawamura, M.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: Comparison with the effects of insulin. J. Biol. Chem. 2001, 276, 42818–42825.
- Takei, N.; Inamura, N.; Kawamura, M.; Namba, H.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 2004, 24, 9760–9769.
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494.
- Campbell, L.E.; Wang, X.; Proud, C.G. Nutrients differentially regulate multiple translation factors and their control by insulin. Biochem. J. 1999, 344, 433–441.
- Ishizuka, Y.; Kakiya, N.; Witters, L.A.; Oshiro, N.; Shirao, T.; Nawa, H.; Takei, N. AMP-activated protein kinase counteracts brain-derived neurotrophic factor-induced mammalian target of rapamycin complex 1 signaling in neurons. J. Neurochem. 2013, 127, 66–77.
- Casadio, A.; Martin, K.C.; Giustetto, M.; Zhu, H.; Chen, M.; Bartsch, D.; Bailey, C.H.; Kandel, E.R. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 1999, 99, 221–237.
- Carroll, M.; Warren, O.; Fan, X.; Sossin, W.S. 5-HT stimulates eEF2 dephosphorylation in a rapamycin-sensitive manner in Aplysia neurites. J. Neurochem. 2004, 90, 1464–1476.
- Carroll, M.; Dyer, J.; Sossin, W.S. Serotonin increases phosphorylation of synaptic 4EBP through TOR, but eukaryotic initiation factor 4E levels do not limit somatic cap-dependent translation in aplysia neurons. Mol. Cell. Biol. 2006, 26, 8586–8598.
- Garelick, M.G.; Kennedy, B.K. TOR on the brain. Exp. Gerontol. 2011, 46, 155–163.
- Ryskalin, L.; Lazzeri, G.; Flaibani, M.; Biagioni, F.; Gambardella, S.; Frati, A.; Fornai, F. mTOR-dependent cell proliferation in the brain. Biomed. Res. Int. 2017, 2017, 7082696.
- Human, M.P.C. A framework for human microbiome research. Nature 2012, 486, 215–221.
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Front. Integr. Neurosci. 2018, 12, 33.
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140.
- De Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471.
- Diaz Heijtz, R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 2016, 21, 410–417.
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013.
- Gensollen, T.; Blumberg, R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1084–1091.
- Sudo, N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv. Exp. Med. Biol. 2014, 817, 177–194.
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139.
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187.
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020.
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107.
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94.
- Grossman, M.I. Neural and hormonal regulation of gastrointestinal function: An overview. Annu. Rev. Physiol. 1979, 41, 27–33.
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496.
- Oldham, M.C.; Konopka, G.; Iwamoto, K.; Langfelder, P.; Kato, T.; Horvath, S.; Geschwind, D.H. Functional organization of the transcriptome in human brain. Nat. Neurosci. 2008, 11, 1271–1282.
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174.
- Caputo, V.; Sinibaldi, L.; Fiorentino, A.; Parisi, C.; Catalanotto, C.; Pasini, A.; Cogoni, C.; Pizzuti, A. Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS ONE 2011, 6, e28656.
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314.
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25.
- Sun, J.; Wang, F.; Hong, G.; Pang, M.; Xu, H.; Li, H.; Tian, F.; Fang, R.; Yao, Y.; Liu, J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2016, 618, 159–166.
- Spiljar, M.; Merkler, D.; Trajkovski, M. The immune system bridges the gut microbiota with systemic energy homeostasis: Focus on tlrs, mucosal barrier, and scfas. Front. Immunol. 2017, 8, 1353.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Guan, N.L.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158.
- Arentsen, T.; Qian, Y.; Gkotzis, S.; Femenia, T.; Wang, T.; Udekwu, K.; Forssberg, H.; Diaz Heijtz, R. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 2017, 22, 257–266.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781.
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726.
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417.
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055.
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995.
- Kodish, I.; Rockhill, C.; Ryan, S.; Varley, C. Pharmacotherapy for anxiety disorders in children and adolescents. Pediatri. Clin. 2011, 58, 55–72.
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828.
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261.
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264.
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e1.
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177.
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487.
- Szklany, K.; Wopereis, H.; de Waard, C.; van Wageningen, T.; An, R.; van Limpt, K.; Knol, J.; Garssen, J.; Knippels, L.M.J.; Belzer, C.; et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr. Neurosci. 2020, 23, 896–910.
- Firmansyah, A.; Dwipoerwantoro, P.G.; Kadim, M.; Alatas, S.; Conus, N.; Lestarina, L.; Bouisset, F.; Steenhout, P. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac. J. Clin. Nutr. 2011, 20, 69–76.
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801.
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Kadosh, K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy female volunteers are associated with reduced negative bias and the gut bacterial composition. medRxiv 2019, 19011403.
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673.
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghaffari, P.; Zhang, C.; Larsson, E.; Bäckhed, F.; Nielsen, J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015, 11, 834.
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48.
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379.
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143.
- Chang, J.P.; Lane, H.Y.; Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 2014, 20, 5180–5185.
- Deutsch, S.I.; Pepe, G.J.; Burket, J.A.; Winebarger, E.E.; Herndon, A.L.; Benson, A.D. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012, 1439, 96–107.
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: A narrative review. Nutrients 2019, 11, 2029.
