Nanocarbon materials have attracted the interest of researchers due to their excellent properties. Nanocarbon-based flame retardant polymer composites have enhanced thermal stability and mechanical properties compared with traditional flame retardant composites.
1. Introduction
The use of carbon materials has a long history. Many types of carbon materials have been produced, from cheap graphite to expensive diamond, as well as carbon black, which is commonly used in the rubber industry. The development history of nano-scale carbon materials is relatively short, but they have exhibited excellent properties to attract the research community. Whenever a new nanocarbon material is found, it introduces revolutionary changes to the field of materials science and technology. In 1985 [1], fullerenes were first discovered. Fullerene is a kind of 0-D nanocarbon material with free-radical trapping properties. In 1991 [2], a 1-D tubular carbon nanomaterial called carbon nanotubes was discovered. In 2004 [3], graphene, a 2-D layered nanomaterial of a single atom in thickness, was discovered and became relevant due to its excellent properties. All of these materials can be regarded as allotropes formed by a large number of carbon six-membered rings [4]. In addition, there are other nanocarbon materials, such as carbon black and expandable graphite. Both have been used to prepare nanocomposites with strong mechanical properties, increased thermal stability, thermal conductivity, and electrical conductivity and flame retardancy [5,6,7,8].
Polymer materials are widely used in various fields because of their excellent properties. As an aspect to highlight, they are basically prepared by polymerization of organic compounds, so they usually have high flammability, which increases the risk of fire during their lifetime. Consequently, most of the polymer materials need to add flame retardants during the preparation cycle. Most of the traditional flame retardants are required to be added in higher amounts to achieve optimal flame retardancy properties in the basic polymer.
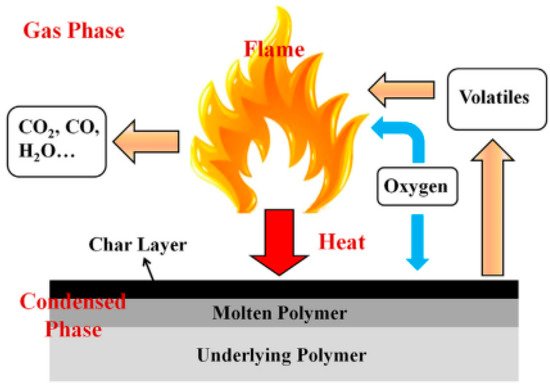
There exist two basic mechanisms to explain the flame retardant property, as illustrated in Figure 1. The first is the mechanism of the gas phase flame retardant that is related to the large number of active free radicals produced during polymer matrix combustion. These free radicals are necessary for the combustion chain reaction as well. Some flame retardants can capture free radicals, leading to the cutting off of the combustion chain reaction. Other flame retardants can produce inert gases, such as NH3, when they are decomposed. This fact permits us to dilute the oxygen and active free radicals, leading to a delay in the development of a flame. The second is the part of the flame retardant that can catalyze the formation of the carbon layer and strengthen it. The carbon layer formed on the substrate can block the contact of heat and oxygen with the underlying matrix material and reduce the diffusion of active free radicals so as to improve the flame retardancy. Most of the carbon nanomaterials can enhance the carbon layer and catalyze the formation of carbon, while others, such as fullerenes, also have the ability to capture free radicals [9].
Figure 1. Illustration of a model of a flame retardant’s action [
9].
2. Graphene
2.1. Synergistic Flame Retardancy of Graphene
Graphene has high thermal stability properties together with physical barrier functions. These characteristics make the graphene have flame retardancy properties to a certain extent. Pristine graphene cannot make a polymer matrix reach proper standard levels of flame retardancy [10]. Consequently, it is a common strategy to use graphene as a synergist in conventional flame retardant systems. We summarize the synergistic flame retardancy of graphene and its modified products in Table 1.
Table 1. Synergistic effect of graphene-based nanomaterials and other flame retardant additives on the flame retardant properties of polymer composites.
| Polymer |
Loading of Graphene Nanomaterials |
Type and Loading of Other Flame Retardant Additives |
Highlights |
Ref |
| EVA |
2 wt% |
ATH 36 wt%, MoS2 2 wt% |
PHRR decreased from 1815 kW/m2 to 377 kW/m2 |
[11] |
| PP |
0.5 wt% |
IFR2 4.5 wt% |
PHRR decreased from 1025 kW/m2 to 140 kW/m2 |
[12] |
| PI |
5 wt% |
MMT 10 wt% |
UL-94: V-0 rating, LOI: 55% |
[13] |
| EP |
2.5 wt% |
DOPO 2.5 wt% |
PHRR reduced from 1194 kW/m2 to 396 kW/m2 |
[14] |
| EP |
7 wt% |
Al2O3 68 wt%, MH 5 wt%, |
UL-94: V-0 rating, LOI: 39% |
[15] |
| PLA |
0.5 wt% |
phosphorus-containing flame retardant 15 wt% |
UL-94: V-0 rating, LOI: 29.2% |
[16] |
| PBT |
0.3 wt% |
IFR 20 wt% |
UL-94: V-0 rating, LOI: 25.4% |
[18] |
| TPU |
0.25 wt% |
MPP 14.75 wt% |
PHRR decreased from 2192.6 kW/m2 to 187.2 kW/m2 |
[21] |
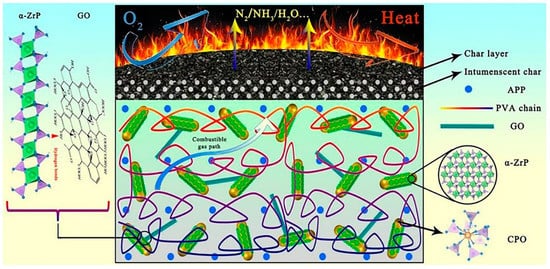
The flame retardant synergistic effect of original graphene is limited. This is an important drawback that typically produces difficulties with meeting the flame retardant demand in most application scenarios. Nonetheless and as previously pointed out, an improved performance flame retardant can be prepared by combining graphene with other materials. Leng et al. [17] prepared a α-zirconium phosphate (α-ZrP)/cerium phosphate (CPO)/graphene oxide (GO) nanocomposite (ZCG) flame retardant. As shown in Figure 2, α-ZRP links graphene oxide and cerium oxide through hydrogen bonds to form a synergistic effect, which improves the dispersion of the flame retardant. Compared with the composites with 10 wt% APP, the LOI of the composites increased from 28% to 36% after replacing the same amount of app with 5 wt% ZCG. At the same time, without adding graphene oxide, the limiting oxygen index of the composites is lower than 36%, which fully proves the synergistic effect of graphene on flame retardancy.
Figure 2. The possible flame retardant mechanism of ZCG/APP/PVA composites [
17].
Transition metal elements have the ability of catalytic carbonization. It is very useful to combine a transition metal with graphene to form a highly efficient flame-retardant synergist that integrates catalytic carbonization and a physical barrier.
Natural fiber-reinforced polypropylene has excellent properties, but its high flammability limits its application. The question of how to enhance its flame retardancy is a very important subject. Although APP, magnesium hydroxide (MH), and other traditional flame retardants can provide better flame retardancy, their addition amount is usually large. By adding exfoliated graphene nanosheets as a flame retardant synergist, the flame retardant performance of traditional flame retardants can be improved. Graphene nanosheets achieve flame retardancy and a synergistic effect mainly through a physical barrier and the promotion of carbonization [
22].
Ionic liquid is a kind of solvent and material in line with the concept of green chemistry. It has high thermal stability and low flammability. It is a compound with great potential to become an excellent flame retardant.
2.2. Inorganic Hybrid Graphene
Graphene is usually used as a flame retardant synergist in composites, but it is difficult to use it as a flame retardant alone. However, based on its excellent properties, it would be useful to prepare a highly efficient flame retardant by the modification of graphene. Liu et al. [
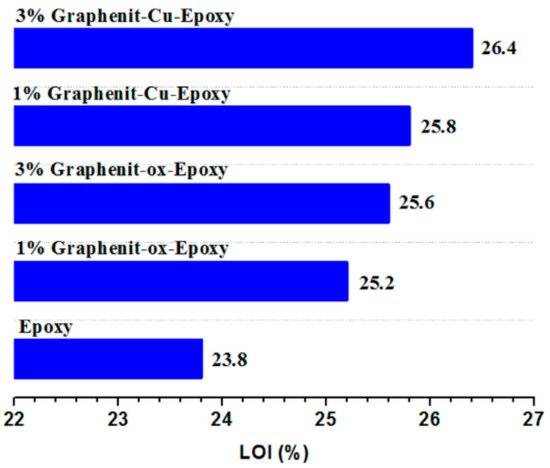
24] successfully prepared copper-doped graphene and applied it to a flame retardant epoxy resin. According to
Figure 4, after adding 3 wt% copper-doped graphene, the LOI of the EP composites increased to 26.4% compared with neat EP. On the other hand, the peak heat release rate of the composites decreased by about 34%, and the smoke suppression performance also improved. In addition, the thermomechanical properties of the composites also changed.
Figure 4. LOI of epoxy resin composites modified by copper-doped graphene [
24].
Reduced graphene oxide/aluminum hypophosphite (AHP/RGO) hybrid materials were prepared by a one-pot method to improve the flame retardancy of aluminum hypophosphite. The cone calorimetric test results of a PBT composite with 20% AHP/RGO show that the PBT/20 wt% AHP/RGO composites can not only reduce the heat release rate but also reduce the release of CO and toxic gas when the material is burned compared with the addition of aluminum hypophosphite alone [
25].
In another direction, black phosphorene (BP) has a unique two-dimensional structure and is a phosphorus-containing compound that has the potential to become a highly efficient flame retardant. However, the mechanical properties of the polymer will deteriorate when black phosphorene is added to the polymer matrix.
The flame retardancy of fabrics and fiber materials has become an important research topic with interesting implications. Most flame retardants added to the fiber will affect the fiber’s color and reduce the mechanical properties.
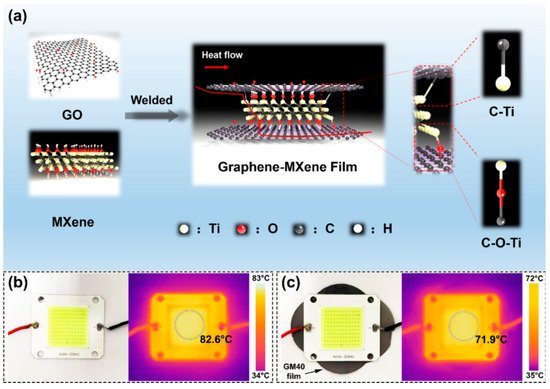
MXene is a two-dimensional transition metal carbide, nitride, or nitrogen carbide. MXene has two-dimensional lamellar structure similar to that of graphene and high thermal conductivity. Consequently, it is considered to be a material with promising thermal conductivity and flame retardant properties. Liu et al. [
28] prepared MXene/graphene nanocomposite films by reduction and thermal welding at a suitable temperature.
Figure 5a shows a model of the interaction between MXene and graphene.
Figure 5b,c indicate the interesting performances of the MXene/graphene nanocomposite as a heat dissipation material for electronic components.
Figure 5. (
a) Combination mode of MXene and graphene (
b) Optical image of the LED and the corresponding IR thermal image. (
c) Application of the GM paper as an ultra-thin heat sink for LED and the corresponding IR thermal image [
28].
Layered double hydroxides are a kind of nano filler that have a high aspect ratio. The natural agglomeration phenomenon requires us to study the surface modification of layered double hydroxides to improve their dispersion.
Mesoporous zinc ferrate (MZF) is a material with high flame retardant potential because of its porous structure and the fact that it contains iron and zinc. Yang et al. [
30] synthesized a mesoporous zinc ferrate hybrid graphene oxide (MZF-GO) composite by a hydrothermal method. By combining the catalytic performance of mesoporous zinc ferrate with the physical barrier function of graphene, improved flame retardancy behavior was obtained. When the addition amount of MZF-GO was 3 wt%, the peak heat release rate of the composite was reduced by 39%. This fact provides a new perspective for the application of an efficient environmental flame retardant.
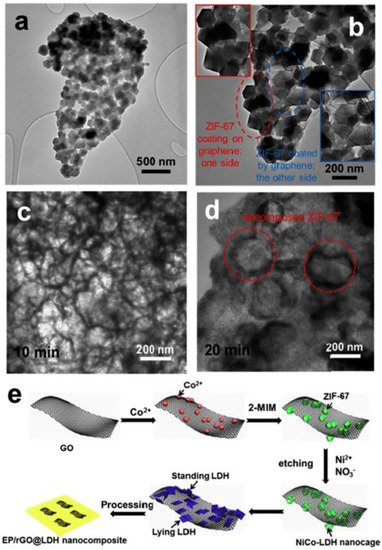
Pan et al. [
31] developed a flame-retardant insulation epoxy resin composite that can rapidly dissipate heat. These properties come from the preparation of nickel–cobalt bimetallic layered hydroxides (NiCo-LDH). As shown in
Figure 6, the acid-sensitive bimetallic organic frameworks are used as sacrificial precursors to form NiCo-LDH on graphene.
Figure 6. (
a) TEM image of rGO@ZIF-67; (
b) High-magnification TEM image of rGO@ZIF-67; (
c,
d) TEM images of the as-synthesized products obtained at different time intervals (10 min (
c), 20 min (
d)); (
e) Schematic illustration of the synthesis of rGO@LDH and the preparation of EP/rGO@LDH nanocomposites [
31].
Aluminum hydroxide (AH) is an important traditional flame retardant; nonetheless, it is added in large amounts (usually 50%), affecting greatly the mechanical properties of pristine materials.
Red phosphorus hybrid graphene nanocomposites were prepared by means of one-step ball milling, leading to a flame-retardant polyimide foam. The composite foam is characterized by a light weight, good mechanical properties, and strong flame retardancy. During the physical hybridization process, red phosphorus forms a phosphate group on the edge of the graphene and forms nanoparticles on the same graphene surface. This makes the red phosphorus hybrid graphene have good dispersion in the polymer matrix and high phosphorus content, which is the basis of its flame retardant properties [
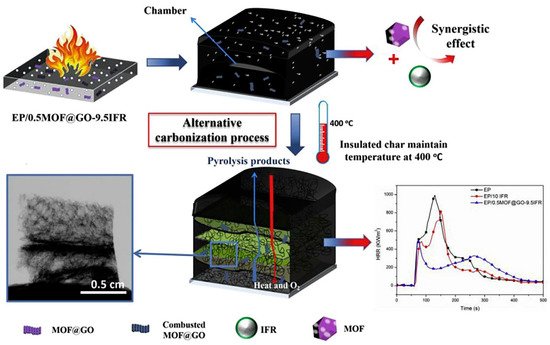
5]. Bimetallic metal–organic framework (MOF) and graphene oxide (GO) nano-hybrids (MOF@GO) have been used as the synergist in intumescent fire retardants (IFRs). According to
Figure 8, these nano-hybrids have a novel alternative dynamic carbonization phenomenon in the accumulated char layer of epoxy resin (EP) composites, and the second heat release rate peak of the EP/0.5 wt%MOF@GO-9.5 wt% IFR was reduced by 59% compared with the EP/10 wt% IFR [
35].
Figure 8. Proposed fire retardancy mechanism of intumescent EP with MOF@GO (EP/0.5MOF@GO-9.5IFR). (A colored version of this figure can be viewed online) [
35].
2.3. Layered Coating of Modified Grapheme
Polyurethane foam is a material with excellent properties; nonetheless, its high flammability may induce limitations towards applications. In addition, many flame retardants will affect the foaming process of polyurethane or reduce the mechanical properties. Kim et al. [
36] used an aqueous liquid crystalline (LC) graphene oxide (GO) scaffold and polydopamine to form a PDA/GO nanocoating on the surface of flexible polyurethane (PU) foam (
Figure 9). The PDA/GO-coated PU foam showed significant flame retardant performance, reflected by a 65% reduction in the PHRR at a 5 wt% PDA/GO loading.
Figure 9. (
a) Scheme for preparing the PDA/GO FR nanocoating on the PU foam assisted by the LC phase of an aqueous GO dispersion. (
b) Polarized optical texture of a concentrated GO dispersion (20 mg mL
−1). (
c) Photograph of the GO LC after extrusion through a pipet into a dopamine solution (2 mg of dopamine mL
−1 in Tris-HCl buffer, pH 8.5). (
d) SEM image of dried (2.5/2.5 wt%) PDA/GO-coated PU foam. The inset is a photograph of the same foam. (
e) Cross-sectional SEM image of the PDA/GO-coated PU foam [
36].
Polydopamine is particularly interesting as it has excellent adhesion and free-radical absorption properties. Using the layer-by-layer self-assembly procedure with the mentioned flame retardants, different coatings can be firmly bonded together and play the role of a gas phase flame retardant.
2.4. Surface Decoration of Graphene
The surface modification of graphene by various methods is also a very effective process that has attracted the attention of researchers and produced numerous studies. Depending on the different modification methods and modifiers, graphene-based composites with different properties can be obtained. Zinc hydroxystannate boxes (ZHS) are a kind of inorganic flame retardant that acts as a smoke suppressor. Li et al. [46] prepared a new flame retardant called GNS-ZHS-M2070 by covalent grafting of polyether amine (M2070) onto the surface of zinc hydroxystannate box-decorated graphene nanosheets (GNS).The GNS-ZHS-M2070/EP-12% composites show excellent flame retardancy. In addition, zinc-hydroxystannate-modified graphene composites were prepared by the hydrothermal reaction of zinc hydroxystannate and graphene oxide in an autoclave. The pHRR of the GNS-ZHS-M2070/EP-12% composite was obviously lower than that of pure epoxy resin. The combination of zinc hydroxystannate and graphene can significantly reduce the amount of smoke emitted during polymer combustion [47].
Through a variety of components capable of modifying graphene’s surface, it is possible to increase the matrix’s material performance up to a level that complies with different demands mainly related to flame retardancy, mechanical properties, and smoke emissions. For instance, graphene was modified by hexachlorocyclotriphosphate (HCCP) and nickel hydroxide to improve the flame retardant properties in both the gas and condensed phases. In virtue of the low addition amount (3 wt%), the loss of mechanical properties is usually small. Furthermore, some researchers have used polyaniline and nickel hydroxide to perform surface modification in the same line as described above to increase the flame retardant properties. Compared with others, composites with these two flame retardants have better smoke suppression performance, which is mainly due to the excellent flame retardancy of nickel hydroxide and grapheme [48,49].
Another important method for modifying the surface of graphene is based on a hydrothermal process. This method is easy to implement and permits us to prepare phosphorus and nitrogen-modified graphene by the hydrothermal reaction of compounds containing phosphorus and nitrogen.
In addition to the above-mentioned surface modification, ZIF-8 was prepared on the surface of graphene oxide by a hydrothermal method. ZIF-8 can catalyze the formation of carbon, which can further improve the barrier effect of graphene, reducing the amount of smoke released as well.
2.5. Organic Phosphorus-Containing Flame Retardant Grafted onto Graphene
The preparation of organophosphorus-modified graphene-based flame retardants by the graft reaction of phosphorus-containing compounds and graphene containing oxidation energy groups on its surface has gradually become an attractive research topic [56]. Table 3 summarizes the flame retardant performance of some polymer nanocomposites based on DOPO-modified graphene.
Table 3. DOPO-modified graphene for different matrix materials.
| Matrix |
Types of Grafting Reaction |
Main Performance |
Ref. |
| 4,4-bismaleimidophenylmethane/2, 2-diallyl bisphenol A (BDM/DBA) resins |
Vinyl trie-thoxy silane and (3-isocyanatopropyl)-triethoxysilane as bridging agents |
UL-94: V-0 rating, LOI: 32.8% |
[57] |
| PUA |
vinyltrimethoxy silane and (3-Isocyanatopropyl)-triethoxysilane as bridging agents |
49% reduction in PHRR observed in cone calorimetry |
[58] |
| PLA |
Reaction of 2,5-dihydroxyphenol with an acyl chloride bond |
83% reduction in TSR observed in cone calorimetry compared with neat PLA |
[59] |
| CF/EP |
Reaction of formaldehyde-modified DOPO with an acyl chloride bond |
PHRR of composites
reduced by 38.9% compared with neat CF/EP |
[60] |
| PS |
Paraformaldehyde, hydroxyethyl acrylate, and POCl3 as bridging agents |
39.1% reduction in PHRR observed in cone calorimetry |
[61] |
3. Carbon Nanotubes
3.1. Pristine Carbon Nanotubes
In accordance with carbon nanotubes’ diameter, two categories can be distinguished: single-walled carbon nanotubes and multi-walled carbon nanotubes (MWCNTs). Multi-walled carbon nanotubes are widely used in composites [
70]. Carbon nanotubes have excellent mechanical and electrical properties and have been studied in many fields, particularly as a mechanical reinforcing filler for polymer materials. The synergistic effects of CNTs in different flame retardant systems are summarized in
Table 4.
Table 4. Synergistic effect of carbon nanotubes on different flame retardant systems.
| Matrix |
Flame Retardant System |
Flame Retardant Performance |
Ref |
| PA66 |
intumescent fire retardant (IFR) |
pHRR and THR were reduced by 76.4% and 76.5%, respectively |
[59] |
| PS |
intumescent fire retardant (IFR) |
pHRR decreased by 30% and LOI: 34.1% |
[72] |
| TPU |
intumescent fire retardant (IFR) |
UL-94: V-0 rating, LOI: 30.1%, pHRR and THR were reduced by 92% and 76%, respectively |
[73] |
| PA6 |
APP |
UL-94: V-0 rating, pHRR decreased by more than 30% |
[75] |
| silicone rubber (SR) |
DHCP-PA (a high phosphorus content flame retardant system) |
UL-94: V-0 rating, LOI: 28.4% |
[76] |
Epoxy resin composites filled with thermally oxidized carbon nanotubes were prepared by a casting method, and their flame retardancy was studied by cone calorimetry. In the case of the addition of 1 wt%, compared with the original carbon nanotubes, the thermal-oxidation-treated carbon nanotubes have better flame retardancy properties [
71]. The synergistic effect of CNTs on polystyrene electromagnetic shielding material with an intumescent flame retardant was also investigated [
72]. The results show that the addition of carbon nanotubes can improve the efficiency of the intumescent flame retardant. Note that when the amount of intumescent flame retardant is greater than 10 wt%, the addition of 1 wt% carbon nanotubes induces better flame retardant performance. The effect of carbon nanotubes on the flame retardancy of thermoplastic polyurethane electromagnetic shielding materials with intumescent flame retardants was also investigated [
73].
3.2. Surface-Functionalized Carbon Nanotubes
The combination of carbon nanotubes and inorganic flame retardants has also attracted research interest. A zinc–aluminum layered double hydroxide/carbon nanotube hybrid (CNT/ZnAl-LDH) was prepared by the coprecipitation method. Transmission electron microscopy (TEM) showed that the CNTs were embedded between the piles of layered double hydroxides. When the amount of CNT/ZnAl-LDH was 2.1 wt%, the flame retardancy of the PU foam was better. The quality of the carbon residue was improved and the flame retardancy was enhanced by the insertion of carbon nanotubes [
83].
The hybridization of organic Fe–Ni layered double hydroxides and carbon nanotubes also yields an effective flame retardant. The dispersion of layered double hydroxides and the carbonization performance of matrix materials can be improved by their hybridization. The results show that the heat release rate and the smoke release rate of composites with only 4 wt% nano fillers had greatly decreased [
84]. Multi-walled carbon nanotubes (MWCNTs) were treated with phosphoric acid, and phosphorus was introduced onto the surface of the CNTs to prepare a new type of phosphorus carbon nanotube (P-MWCNT) flame retardant. A polystyrene system was found to have better flame retardancy by compounding 10 wt% P-MWCNTs into the polystyrene. This may be related to the dispersion of carbon nanotubes [
85].
A new type of flame retardant was prepared by the polymerization of hexachlorophosphine cyanogen and p-phenylenediamine and coating with the resulting polymer the surface of carbon nanotubes (PCP-CNT), which were used to modify the flame retardancy of PBT.
APP was modified with a silane coupling agent, coated by carbon nanotubes, and named CAPP. It was also used for the flame retardant modification of PBS.
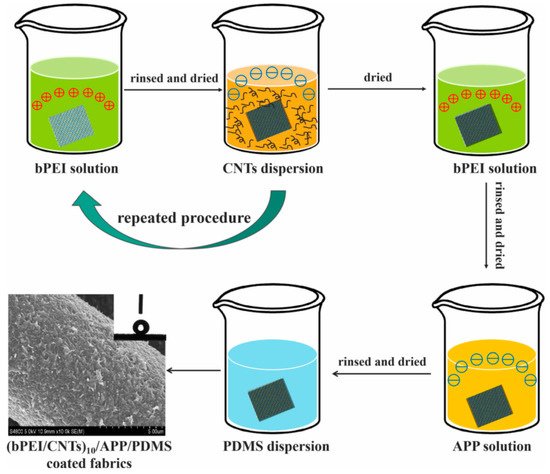
Flame-retardant cotton fibers were prepared by sequential self-assembly of polyethylenimine, ammonium polyphosphate, and carbon nanotubes on the surface of cotton fibers (
Figure 16) [
88].
Figure 16. Schematic illustration of the fabrication of multifunctional superhydrophobic fabrics [
88].
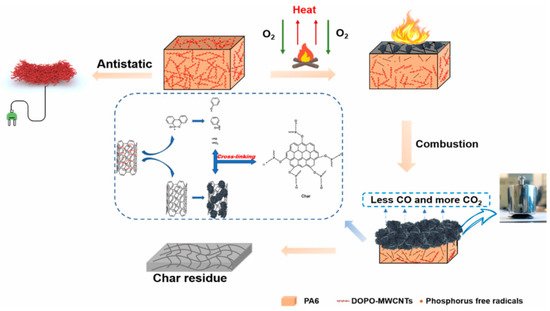
The possible flame retardant mechanism of DOPO-modified CNTs is briefly summarized in
Figure 17 [
90].
Figure 17. Possible flame retardant and antistatic mechanism of PA6/DOPO multi-walled carbon nanotubes (MWCNTs) [
90].
4. Fullerene and Other Nanocarbons
Fullerene is an allotrope of carbon. It has a hollow sphere structure, which is usually composed of six-membered rings at a number ranging from five to seven. The free radical trapping effect of fullerenes can improve the flame retardancy of materials and has attracted the attention of many researchers [
95]. In addition, other types of carbon nanomaterials, such as expandable graphite and carbon black, have been a source of research as well.
Song et al. [
96] prepared a low nitrogen and phosphorus content intumescent flame retardant (PDBPP) and grafted it onto fullerene spheres to prepare a c60-d-PDBPP integrated flame retardant, which was used for the flame retardant modification of PP.
Fullerene may affect the cross-linking of high-temperature vulcanized materials, such as silicone rubber, resulting in a decline in the material’s performance. A modified fullerene was prepared by the combination of polymethylphenylsiloxane and fullerene by the stacking effect (PMPS-d-C60). By adding 4 phr pmps-d-c60 to the silicone rubber, the LOI reached 29.0%, showing excellent flame retardancy [
99]. The combination of DOPO and fullerene is also an important flame retardant modification method. Following this approach, it is possible to enhance the free radical capture ability of fullerenes and prolong the ignition time. By adding 3 wt% DOPO-d-C
60, the peak of the heat release rate of PP composites was decreased slightly [
100].
Expandable graphite (EG) is a low-cost, one-component intumescent flame retardant and also a kind of nano carbon material. When the content of expandable graphite, huntite, and hydromagnesite is 25 wt%, the TPU composite reaches the V-0 level in the vertical combustion test and the highest limiting oxygen index. With the increase in the expandable graphite content, the mechanical properties of TPU composites are improved, which overcomes the disadvantage of the mechanical properties of TPU composites due to the addition of the inorganic flame retardant [
101].
Carbon black (CB) is also an important nano carbon material. In most cases, carbon black appears as a reinforcing modifier of rubber. However, carbon black can be used as a synergist to improve the flame retardant efficiency of other flame retardant materials, especially magnesium hydroxide. The addition of 0.5 wt% carbon black can make EVA/MH composites reach the V-0 level in the vertical combustion test and improve the mechanical properties of the matrix material to a certain extent [
104].
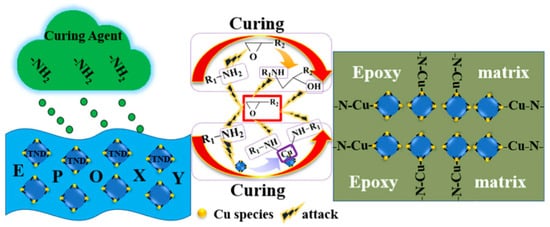
Nanodiamond (ND) was modified with P species, Cu species, and (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) step by step in order to prepare a flame retardant and strengthen the mechanical properties of nano carbon materials for epoxy composites. Nanodiamond was used as a carrier to connect copper, tempo resin, and epoxy resin. The mechanical properties and flame retardancy of the composites were further enhanced by forming an interconnected network with the epoxy resin matrix during the curing process.
Figure 21 shows that the coupling agent on the surface of the nanodiamond participates in the curing process of epoxy resin. The nanodiamond can form a reinforcing network in the epoxy resin matrix [
106].
Figure 21. Proposed mechanism for the reinforcing effect by TND [
106].
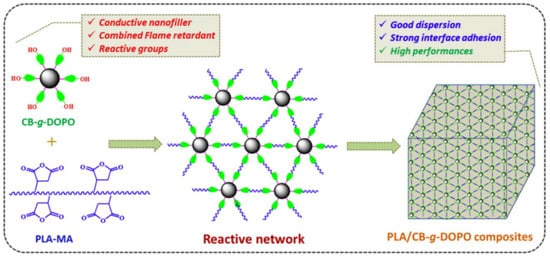
DOPO (9,10-dihydro-9-oxy-10-phosphazene-10-oxide) is an effective phosphorus-containing flame retardant. However, DOPO easily migrates to the polymer matrix material, which reduces the flame retardant’s efficiency. By introducing carbon black as the basis of a modification, DOPO was grafted onto the surface of hydroxylated carbon black to prepare hybrid materials (CB-g-DOPO) with a high grafting rate. The PLA/8 wt% CB-g-DOPO composite not only has strong flame retardancy but also shows a certain degree of conductivity. As shown in
Figure 22, the CB-g-DOPO reacted with maleic anhydride in PLA-MA. This reaction realizes the combination of the modifier and the matrix, hinders the diffusion of the flame retardant, and improves the dispersion of the flame retardant [
107].
Figure 22. Schematic illustration of the chemical reaction between CB-g-DOPO and PLA-MA to form a reactive network in the PLA matrix [
107].
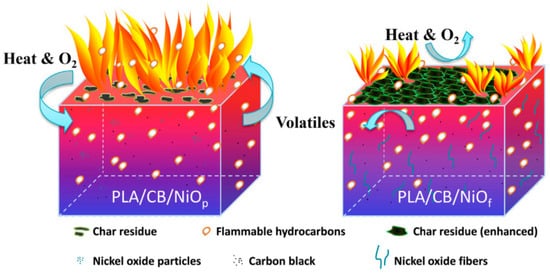
Zhang et al. [
108] fabricated porous submicron nickel oxide (NiO) fibers via an electrospinning and subsequent pyrolysis process. Carbon black (CB) can also be combined with NiO to form a reticulated flame retardant system and plays the part of a connection point in this system. The presence of carbon black enhances the carbonization ability of NiO. CB/NiO
f mainly plays the role of a condensed-phase flame retardant in PLA’s combustion (
Figure 23).
Figure 23. Proposed flame retardant mechanism for PLA composites with carbon black and NiO particles/fibers [
108].
5. Summary and Perspective
In recent years, nano carbon-based materials have been widely used to modify polymers with flame retardants. Compared with traditional flame retardants, nano carbon-based materials can significantly improve the flame retardancy, mechanical properties, and thermal and electrical conductivities of multi-functional flame-retardant nanocomposites. Most carbon nanomaterials have special shapes, which are closely related to their excellent properties. Moreover, nano carbon-based materials can be used in a variety of matrix materials or directly made into single-component materials with certain physical properties. Some researchers [
81] used carbon nanotubes as the matrix to prepare paper, which provides a new idea for the preparation of novel nanocomposites.
The mechanism of nano carbon materials as flame retardants in different polymer matrices is similar to that of traditional flame retardants. There are two main ways to delay the combustion of the polymer matrix:
(I) The addition of nano carbon material, which helps to form a dense protective carbon layer. Adding nano carbon materials leads to a more compact carbon layer formed by combustion (with fewer cracks and holes). These carbon layers can protect the polymer material at the bottom and block the combustible gas generated so as to protect the matrix material.
(II) Some nano carbon materials, such as fullerenes, have the ability to capture free radicals. They absorb the active free radicals produced during combustion and terminate the chain reaction.
Due to their limited function as a flame retardant, in most cases pristine nano carbon materials are used as synergists together with traditional flame retardants. Moreover, obtaining a good dispersion of pristine carbon nanomaterials in the polymer matrix remains challenging. A poor dispersion of carbon nanomaterials may lead to a deterioration of the mechanical properties. In order to avoid these shortcomings, the surface modification of carbon nanomaterials can be performed. There are two goals in this modification: (i) to reduce the interaction between the carbon nanomaterials and improve their dispersion in the polymer matrix; and (ii) to introduce functional groups in the molecular chains with flame retardancy.
Although some nano carbon materials have excellent properties, the development of low-cost preparation methods for most of the carbon nanomaterial remains challenging. These facts make nano carbon materials difficult to apply on a large scale. We expect that more and more researchers will contribute to low-cost production methods for nano carbon materials. Moreover, the surface functionalization of carbon nanomaterials is quite important to flame retardant applications and will be one of the main research topics in the future. In addition, to date, few researchers have focused on the recyclability of carbon nanocomposites. With the increasing awareness of environmental protection and energy conservation, it would be very important to improve the recyclability of materials and, in particular, develop a new strategy for recycling thermoset-based carbon nanocomposites. An interesting approach would be the use of waste thermoset-based carbon nanocomposites to prepare high-value-added carbon materials.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26154670