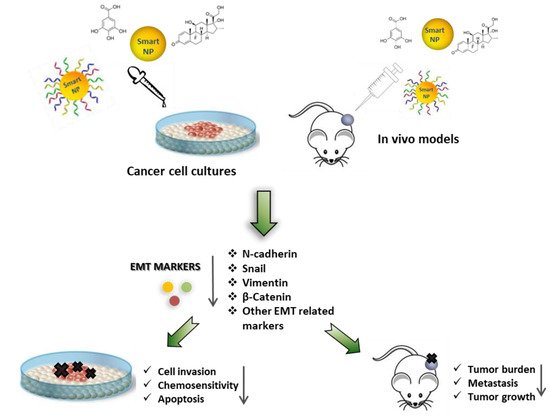
Epithelial-mesenchymal transition (EMT) has emerged as a key regulator of cell invasion and metastasis in cancers. However, although EMT represents a relevant therapeutic target for cancer treatment, its application in the clinic is still limited due to various reasons, including appropriate drug delivery. Different nanomaterials may be used to counteract EMT induction, providing novel therapeutic tools against many different cancers. We discuss the application of various nanomaterials for EMT-based therapies in cancer, the therapeutic relevance of some of the proposed EMT targets, and the potential benefits and weaknesses of each approach.
- nanomaterials
- nanomedicine
- epithelial-mesenchymal transition
- cancer metastasis
- cancer therapy
1. Introduction
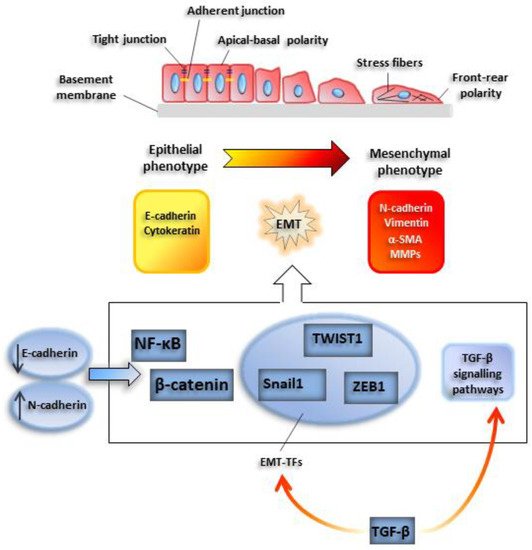
2. Epithelial to Mesenchymal Transition: From Physiology to Cancer
2.1. EMT-TFs
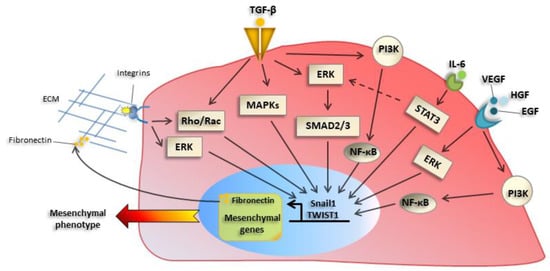
2.2. Extracellular Inducers of EMT: Role of TGFβ
2.3. Noncoding RNAs
2.4. Specificities of Tumor EMT
3. Nanomedicine in Cancer Therapy
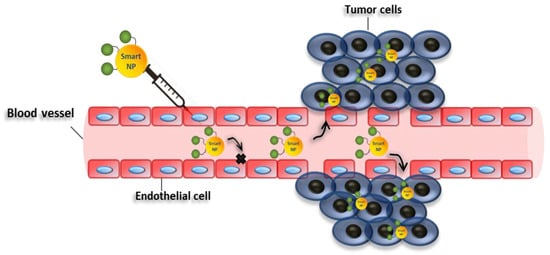
4. Targeting EMT with Nanoparticles for Cancer Therapy
| Entry | NPs | Combined Treatments | Target Cells | Cancer Tissues | Regulation of EMT Markers | Biological Effects | Refs |
|---|---|---|---|---|---|---|---|
| 1 | Gold nanoparticles | none | A2780, OVCAR5 SKOV3-ip |
Epithelial ovarian cancer | E-cadherin↑ N-cadherin↓ Snail↓ p42/44↓ MAPK↓ |
Inhibition of cell proliferation; Reversion of epithelial plasticity; Inhibition of EMT |
[48] |
| 2 | Gold nanoparticles | none | A2780, OVCAR5 and SKOV3-ip |
Epithelial ovarian cancer | ALDH1↓, CD44↓, CD133↓, Sox2↓, MDR1↓, ABCG2↓ Akt signaling↓ NF-κB signaling↓ E-Cadherin↑ β-Catenin↑ Vimentin↓ α-SMA↓ |
Sensitivity to cisplatin; Suppression cancer stem cell proprieties; Inhibition of EMT |
[49] |
| 3 | Gold nanoparticles | none | PANC-1, AsPC-1 and HPAF II | Pancreatic cancer | E-cadherin↑ N-cadherin↓ Vimentin↓ |
Sensitivity to gemcitabine; Suppression cancer stem cell proprieties; Inhibition of EMT |
[50] |
| 4 | Gold nanoparticles | none | HUVECs B16F10 |
Melanoma Blood vessels |
E-Cadherin↑ ZO-1↑ Vimentin↓ C-myc↓ MMP2↓ |
Inhibition of cell migration; Inhibition of EMT |
[51] |
| 5 | Gold nanorods | none | HeLA MCF-7 |
Cervical cancer Breast cancer |
Vimentin↓ N-cadherin↓ |
Inhibition of collective migration; Decrease of EMT markers | [52] |
| 6 | Gold Nanoparticles | Cold plasma | T98G A459 |
Glioblastoma Lung cancer |
E-Cadherin↑ N-Cadherin↓ Slug↓ ZEB1↓ PI3K/AKT patwhay ↓ |
Apoptosis; Reduction of cell proliferation; Inhibition of EMT; Decrease in sphere formation; Decrease in self-renewal capacity |
[53] |
| 7 | Titanium dioxide | none | A459 | Epithelial lung cancer | Smad2/3↓ E-Cadherin↑ N-cadherin↓ |
Inhibition of TGF-β-Mediated Cell Migration; Suppression of TGF-β-Induced EMT; Attenuation of TGF-β Signaling |
[54] |
| 8 | Titanium dioxide Silicon dioxide |
none | LX-2 | Fibrosis | N-Cadherin↓ E-Cadherin↑ |
Inhibition of EMT; Inhibition of fibrosis; Reduction of adhesion and migration profiles |
[55] |
| 9 | ZnO Nanostructures | none | T98G SNU-80 H-460 |
Glioblastoma Thyroid cancer Lung cancer |
N-Cadherin↓ ZEB1↓ |
Cell death; Apoptosis; Reduction of cell invasion; Inhibition of EMT |
[56] |
| 10 | D, L-lactic-co-glycolic acid (PLGA) | α–Mangostin | PANC-1, AsPC-1, MIA PaCa-2; Human CSCs, KrasG12D mouse CSCs |
Pancreatic cancer | E-cadherin↑ N-cadherin↓ Slug, Snail1, ZEB1↓ Nanog, c-Myc, Oct4↓ Shh pathway↓ Gli targets↓ |
Inhibition of cancer growth; development; metastasis; inhibition of pluripotency; Inhibition of EMT |
[57] |
| 11 | D, L-lactic-co-glycolic acid (PLGA) | Anthothecol | PANC-1, AsPC-1, MIA PaCa-2; Human CSCs, KrasG12D mouse CSCs |
Pancreatic cancer | E-cadherin↑ N-cadherin↓ Slug, Snail, ZEB1↓ Nanog, c-Myc, Oct4↓ Shh pathway↓ Gli targets↓ |
Inhibition of cell proliferation; invasion; migration; induction of apoptosis; inhibition of pluripotency; Inhibition of EMT |
[58] |
| 12 | D, L-lactic-co-glycolic acid (PLGA | Wedelolactone | MDA-MB-231 Breast cancer stem cells |
Triple negative breast cancer | E-Cadherin↑ N-Cadherin↓ TWIST1↓ Snail↓ Vimentin↓ |
Retarded migration and invasion; Reduction of cell viability; Apoptosis; Inhibition of EMT; Reduction of pluripotency; Drug sensitivity to paclitaxel |
[59] |
| 13 | D, L-lactic-co-glycolic acid (PLGA) | Salinomycin | AsPC-1 | Pancreatic cancer | E-Cadherin↑ β catenin↑ TGFβ R-1↓ TGFβ R-2↓ |
Inhibition of EMT; Apoptosis |
[60] |
| 14 | Polymeric micelles | Salinomycin | A459 | Lung cancer | Vimentin↓ | Inhbition of EMT; Reversion to epithelial phenotype; Reduction of cell migration; Prevention of P-gp efflux |
[61] |
| 15 | Silver nanoparticles | Gallic Acid | A459 | Lung cancer | Vimentin↓ N-cadherin↓ Snail1↓ E-cadherin↑ |
Loss of radiation-induced metastasis; Inhibition of EMT |
[62] |
| 16 | Curcumin loaded selenium nanoparticles (Se-Cu NPs) | Curcumin | HCT116 | Colon cancer | CD44↓ N-Cadherin↓ |
Induction of autophagy; Induction of apoptosis; Induction of cell cycle arrest; Inhibition of EMT |
[63] |
| 17 | Curcumin loaded selenium nanoparticles (Se-Cu NPs); CD44-targeted DOX loaded nanoparticles (PSHA-DOXNPs) |
Curcumin, Doxorubicin |
HCT116 | Colon cancer | N-Cadherin↓ Vimentin↓ Snail1↓ CD44↓ MMP2↓ MMP4↓ |
Induction ROS levels; Decreased mitochondrial membrane potential; Induction cell cycle arrest; Apoptosis; Inhibition of EMT |
[64] |
| 18 | Gold Nanoparticles | Quercetin | MCF-7 MDA-MB-231 HUVECs |
Breast cancer | E-Cadherin↑ N-Cadherin↓ Vimentin↓ Snail↓ Slug↓ TWIST1↓ MMP2/9↓ EGFR/VEGFR-2 signalling↓ |
Inhibition of EMT; Inhibition of angiogenesis; Inhibition of cell invasion |
[65] |
| 19 | Liposomal | Quercetin | Eca109/9706 | Esophageal squamous cell carcinoma |
E-Cadherin↑ | Apoptosis; Inhibition of EMT |
[66] |
| 20 | Mesoporous silica; PEG-PLA micelles | Epigallocatechin gallate/iron | 4T1 | Mouse breast cancer | MMP2/9 ↓ VEGF↓ Vimentin↓ E-cadherin↑ |
Suppression of metastasis; Inhibition of EMT |
[67] |
| 21 | Layered double hydroxide | Etoposide | U87MG Glioblastoma stem cells (GSCs) |
Glioblastoma | Sox2↓ Oct4↓ Nanog↓ Nestin↓ Snail↓ N-Cadherin↓ E-Cadherin↑ PI3K/AKT/mTOR↓ WNT/GSK3β/β-catenin↑ |
Inhibition of cell proliferation; Down-regulation of GSCs stemness; Inhbition of EMT |
[68] |
| 22 | Liposomes | ADH-1 peptide Paclitaxel |
MCF7-paclitaxel resistant | Breast cancer | N-Cadherin↓ E-Cadherin↑ |
Improvement of chemosensitivity; Inhibition of cell migration; Inhibition of EMT |
[69] |
| 23 | Liposomes | ADH-1 peptide DOX Hyaluronic Acid |
A459 | Lung cancer | N-Cadherin↓ CD44↓ |
Drug sensitivity; Reduction of cell migration; Inhibition of EMT |
[70][71] |
| 24 | Gold nanoparticles | Dexamethasone (DSH) thiol derivative Withaferin (WFA) |
B16F10 | Murine melanoma | E-Cadherin↑ Vimentin↓ pAKT/AKT signalling↓ |
Induction of apoptosis; Inhibition of cell cycle; Induction of MET; Inhibition of EMT |
[72] |
| 25 | Zinc arsenite | Arsenic trioxide | Hep3b, HepG2, Bel7402 and MHCC97L | Liver cancer | E-Cadherin↑ Vimentin↓ Slug↓ SHP-1/JAK2/STAT3↓ |
Suppress tumor initiation and growth; Suppression metastasis Inhibition stemness and EMT |
[73] |
| 26 | Albumin based nanoparticles | Arsenic trioxide | in 5-8F CNE-2 |
Nasopharyngeal carcinoma | E-Cadherin↑ N-Cadherin↓ Vimentin↓ |
Inhibition of colony formation; Inhibition of cell invasion; Inhibition of EMT |
[74] |
| 27 | Liposome | 188Re | ES-2-luc | Ovarian cancer | E-Cadherin↑ Vimentin↓ p53↑ |
Switch to mitochondrial phosphorylation; Reactivation of p53 function; Inhibition of EMT |
[75] |
| 28 | Liposome | 188Re | FaDu | Head and neck squamous cell carcinoma | Let-7↑ | Suppression of tumor growth | [76] |
| 29 | Liposome | 188Re | FaDu, SAS |
Head and neck squamous cell carcinoma | E-Cadherin↑ N-Cadherin↓ TWIST1/2 ↓ Vimentin↓ ZEB1↓ Slugs↓ |
Inhibition of cell proliferation; Cell death; Inhibition of EMT |
[77] |
| 30 | Liposome | Simvastatin, Paxicitel | A549T PC9 TAM (tumor associated macrophages) |
Lung and prostate cancer | FAK↓ ERK/AKT↓ TNF-α↑ TGFβ↓ LXR/ABCA1↓ E-Cadherin↑ Vimentin↓ |
Inhibition of EMT; Sensitization to paxicitel; Repolarization of TAM; Regulation of cholesterol metabolism |
[78] |
| 31 | Carboxymethyl dextran (CMD)-chitosan nanoparticles (ChNPs) | Snail siRNA DOX |
HCT-116 | Colon cancer | MMP9 ↓ Vimentin↓ E-cadherin↑ |
Inhibition cell growth; apoptosis; inhibition of migration; Inhibition of EMT |
[79] |
| 32 | Carboxymethyl dextran (CMD)-chitosan nanoparticles (ChNPs) | Snail siRNA SN38 |
PC-3 | Prostate cancer | E-cadherin↑ Claudin-1↑ |
Reduction of cell proliferation; Reduction of cell migration; Inhibition of EMT |
[80] |
| 33 | Carboxymethyl dextran (CMD)-chitosan nanoparticles (ChNPs) | Snail siRNA HMGA2 siRNA DOX |
A459 | Lung cancer | HMGA2↓ E-cadherin↑ Vimentin↓ MMP9↓ |
Apoptosis; Reduction in cell migration; Drug sensitivity; Inhibition of EMT |
[81] |
| 34 | Polypeptide micelles (PEG–PLL–PLLeu) | ZEB1 siRNA DOX |
H460 | Non-small cell lung cancer (NSCLC) | ZEB1↓ E-cadherin↑ SOX2↓ ABCG2↓ |
Inhibition of EMT; Repression of CSC properties; Reduction of cell invasion; Sensitivity to DOX |
[82] |
| 35 | Polyamidoamine dendrimers (PAMAM) and Hyaluronic-acid conjugated mesoporous silica nanoparticles (MSN-Has) | TWIST1 siRNA Cisplatin |
F2 Ovcar8 |
Ovarian cancer | Vimentin↓ E-Cadherin↑ N-Cadherin↓ |
Chemosensitivity to cisplatin; Inhibition of EMT |
[83][84] |
| 36 | Mesoporous Silica | TWIST1 siRNA | MDA-MB-435S | Melanoma | Vimentin↓ CCL2↓ |
Inhibition of migration; Inhibition of EMT |
[85] |
| 37 | Chitosan-coated nanoparticles | TWIST1 siRNA | CNE2 | Nasopharyngeal carcinoma | p-ERK↑ | Sensitivity to radiation; Irradiation-induced apoptosis |
[86] |
| 38 | Polyamidoamine dendrimers (PAMAM) | TWIST1 siRNA | SUM1315 | Triple negative breast cancer | N-Cadherin↓ Vimentin↓ |
Reduction of cell migration and invasion; Inhibition of EMT |
[87] |
| 39 | (PLGA)2-PEI-DMMA nanoparticles | NgBR siRNA | HUVECs MDA-MB-231 4T1 |
Breast cancer | Vimentin↓ E-Cadherin↑ |
Inhibition of endothelial cell migration; Suppression of cancer cell invasion Normalization of tumor blood vessel; Inhibition of EMT |
[88] |
| 40 | ECO lipid carrier | β3 integrin siRNA | MDA-MB-231 | Triple negative breast cancer | PAI-1↓ N-cadherin↓ E-cadherin↑ CK19↑ |
Inhibition of TGFβ-mediated cytostasis; Inhibition of TGFβ-mediated EMT; Inhibition of TGFβ-mediated invasion; Inhibition of 3-dimensional organoid growth; Inhibition of EMT |
[89] |
| 41 | ECO lipid carrier | DANCR siRNA | MDA-MB-231 BT549 |
Triple negative breast cancer | β-catenin↓ ZEB1↓ Stat proteins↓ N-cadherin↓ Survivin↓ WNT signaling↓ |
Inhibition of cell invasion; Inhibition of cell migration; Reduction of survival; Reduction in tumor spheroid Formation; Inhibition of cell proliferation Inhibition of EMT |
[90] |
| 42 | Poly(lactide-co-glycolide) acid nanoparticles (PLGA NPs) | DCAMKL-1 siRNA | HCT116 | Colon cancer | miRNA 200a↑ miRNA let-7a↑ E-Cadherin↑ ZEB1/2↓ Snail↓ Slug↓ |
Inhibition of tumor growth; Inhibition of EMT |
[91] |
| 43 | Polyethylene glycol-polyethyleneimine-chlorin e6 | Wnt-1 siRNA | KB | Oral squamous cell carcinoma | Wnt-1 ↓ β-catenin↓ Vimentin↓ |
Inhibition of cell growth; sensibility to PDT; Inhibition of EMT |
[92] |
| 44 | Cationic solid lipid nanoparticles (SLN) | STAT3 decoy oligodeoxynucleotide | A2780 SKOV3 |
Ovarian cancer | E-Cadherin↑ Snail↓ MMP9↓ |
Induction of cell death; Apoptotic and autophagy cell death; Inhibition of invasion; Inhibition of EMT |
[93] |
| 45 | Gelatin nanoparticles | AXL siRNA | H820 and H1975 erlotinib-resistant | Non-small cell lung cancer | MMP9↓ MMP2↓ Vimentin↓ N-cadherin↓ |
Overcome of chemoresistance to tyrosine kinase inhitors; Inhbition of EMT |
[94] |
| 46 | Polyethylene glycol–polyethylenimine–magnetic iron oxide (PEG-PEI-IONPs) | microRNA-21 antisense oligonucleotides; Gemcitabine |
PANC-1 MIA PACA-2 |
Pancreatic cancer | PTEN↑ PDCD4↑ Vimentin↓ E-Cadherin↑ |
Inhibition of cell proliferation; Apoptosis; Inhibition of invasion and cell migration; Inhibition of EMT |
[95] |
| 47 | Lipid–polymer hybrid nanoparticles modified with CPP | Afatinib; miR-139 |
Caco-2 | Colorectal cancer | E-Cadherin↑ β-catenin↓ Slug↓ |
Inhbition of EMT; Sensitivity to afatinib; Reduced cancer cell migration |
[96] |
| 48 | Gelatinases-stimuli poly(ethylene glycol)-peptide-poly(ε-caprolactone) copolymer | miR-200c Docetaxel |
BGC-823 CSCs and non-CSCs |
Gastric adenocarcinoma | E-Cadherin↑ CD44↓ |
Drug sensitivity to DOC; Inhibition of EMT |
[97] |
4.1. Modulating EMT with Unmodified Nanomaterials
4.1.1. Gold Nanoparticles
4.1.2. Metal Oxide Nanoparticles
4.2. Delivery of Small Molecules to Inhibit EMT
| Entry | NPs | Compound/Drugs Carried | Animal Models | Diseases Model | Biological Effects | Refs |
|---|---|---|---|---|---|---|
| 1 | Gold | none | orthotopic ovarian tumor models (A2780 cells or SKOV3-ip) | Ovarian cancer | Inhibition of tumor growth; Inhibition of metastasis; Inhibition of EMT |
[48] |
| 2 | Gold | none | Female athymic nude mice orthotopic ovarian tumor models (SKOV3-ip) | Ovarian cancer | Drug sensitivity to cisplatin; Reduction of tumor growth; Inhibition of EMT |
[49] |
| 3 | Gold | none | Female C57BL6 mice | Melanoma | Improvement of vascular morphology; Increase vascular perfusion and decrease permeability; Alleviation of hypoxia; Reduction of lung metastasis; Inhibition of EMT |
[51] |
| 4 | Gold Nanoparticles | Cold plasma | Athymic balb/c female nude mice U87MG xenograft model |
Glioblastoma Lung cancer |
Reduction tumor growth | [53] |
| 5 | Titanium dioxide | none | Wild-type zebrafish AB strain; Male C57BL/6 mice |
Caudal regeneration; Colitis |
Impairment of Caudal Fin Regeneration; Colon injury |
[54] |
| 6 | D, L-lactic-co-glycolic acid (PLGA) | α–Mangostin | KC (PdxCre;LSL-KrasG12D); KPC (PdxCre;LSLKrasG12D;LSL-Trp53R172H |
Pancreatic cancer | Inhibition of cancer growth; development; metastasis; Inhibition of EMT |
[57] |
| 7 | D, L-lactic-co-glycolic acid (PLGA) | Wedelolactone | Female Swiss Albino mice | Triple negative breast cancer | Reduction tumor volume; Reduction cancer stem cells |
[59] |
| 8 | D, L-lactic-co-glycolic acid (PLGA) | Salinomycin | Female athymic nude mice AsPC-1-luc orthotopic model |
Pancreatic cancer | Reduction tumor growth; Inhibition of EMT |
[60] |
| 9 | Curcumin loaded selenium nanoparticles (Se-Cu NPs) | Curcumin | Ehrlich’s ascites carcinoma (EAC)-bearing mice. |
Colon cancer | Reduction tumor mass; Increasing mean survival time |
[63] |
| 10 | Curcumin loaded selenium nanoparticles (Se-Cu NPs); CD44-targeted DOX loaded nanoparticles (PSHA-DOXNPs) |
Curcumin, Doxorubicin |
Ehrlich’s ascites carcinoma (EAC)-bearing mice. |
Colon cancer | Decreased tumor burden; Increased survival |
[64] |
| 11 | Gold Nanoparticles | Quercetin | Female Sprague-Dawley rats | Breast cancer | Inhibition of tumor growth; Inhibition of metastasis |
[65] |
| 12 | Mesoporous silica; PEG-PLA micelles | Epigallocatechin gallate/iron | Female balb/c mice |
Breast cancer | Suppression of metastasis; Inhibition of EMT |
[67] |
| 13 | Layered double hydroxide | Etoposide | Female BALB/c nude mice | Glioblastoma | Reduction of tumor growth; Induction of apoptosis; Inhibition of pluripotency; Inhibition of EMT |
[68] |
| 14 | ADH-1 peptide-modified liposomes | Paclitaxel | Female BALB/c nude mice | Breast cancer | Inhibition of tumor growth; Enhancement of chemosensitivity |
[69] |
| 15 | Gold nanoparticles | Dexamethasone (DSH) thiol derivative Withaferin (WFA) |
Old female C57BL/6J mice | Melanoma | Induction of apoptosis; Inhibition of tumor growth; Reduction in mice mortality; Inhibition of EMT |
[72] |
| 16 | Albumin based nanoparticles | Arsenic trioxide | Xenograft model | Nasopharyngeal carcinoma | Reduction tumor growth; Inhibition of EMT |
[74] |
| 17 | Liposome | 188Re | Balb/C nude mice Orthotopic xenograft model | Ovarian cancer | Reactivation p53; Switch to oxidative phosphorylation; Inhibition of EMT |
[75] |
| 18 | Liposome | 188Re | Balb/C nude mice Orthotopic xenograft model | Head and neck squamous cell carcinoma | Suppression of tumor growth | [76] |
| 19 | Liposome | 188Re | Balb/C nude mice Orthotopic xenograft model | Head and neck squamous cell carcinoma | Suppression of tumor growth; Survival; Inhibition of EMT; Inhibition of proliferation markers; Accumulation in tumor site |
[77] |
| 20 | Liposome | Simvastatin, Paxicitel | A549T xenograft mouse model | Lung cancer | Reversion of chemoresistance to PTX in vivo; Arrest of cell growth; Inhibition of EMT; Change of cell polarization of TAM in vivo |
[78] |
| 21 | Polypeptide micelles (PEG–PLL–PLLeu) | ZEB1 siRNA DOX |
Female BALB/c nude mice H460 xenograft model |
Non-small cell lung cancer (NSCLC) | Inhibition of EMT; Repression of CSC properties; Inhibition of metastasis; Inhibition of tumor growth |
[82] |
| 22 | Polyamidoamine dendrimers (PAMAM) and Hyaluronic-acid conjugated mesoporous silica nanoparticles (MSN-Has) | TWIST1 siRNA Cisplatin |
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice | Ovarian cancer | Inhibition of tumor growth; Sensitivity to cisplatin; Inhibition of EMT |
[83][84] |
| 23 | Mesoporous Silica | TWIST1 siRNA | NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) | Melanoma | Reduction of tumor burder; Inhibition of EMT |
[85] |
| 24 | PEI-PDHA PEG-PDHA P85-PEI/TPGS |
TWIST1 siRNA Snail siRNA Paclitaxel |
4T1 tumor-bearing mice models | Breast cancer | Reduction of metastasis; Inhibition of cell invasion; Inhibition of ECM degradation Inhibition of tumor growth |
[111][112] |
| 25 | Polyamidoamine dendrimers (PAMAM) | TWIST1 siRNA | NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice | Breast cancer | Biodistribution in tumor site | [87] |
| 26 | (PLGA)2-PEI-DMMA nanoparticles | NgBR siRNA | Female BALB/c nude mice Orthotopic model |
Breast cancer | Reduction of metastasis | [88] |
| 27 | ECO lipid carrier | β3 integrin | Female nude mice (nu/nu Balb/c background) | Triple negative breast cancer | Reduction of primary tumor burden; Inhibition of metastasis; Inhibition of EMT |
[89] |
| 28 | ECO lipid carrier | DANCR siRNA | Nude athymic mice MDA-MB-231 and BT549 xenograft model |
Triple negative breast cancer | Reduction of tumor growth; Inhibition of EMT |
[90] |
| 29 | Poly(lactide-co-glycolide) acid nanoparticles (PLGA NPs) | DCAMKL-1 siRNA | Male athymic nude mice (NCr-nu/nu) HCT116 cells orthotopic models |
Colon cancer | Arrest in tumor growth; Inhibition of EMT; Regulation of oncogenic miRNAs network |
[91] |
| 30 | Polyethylene glycol–polyethylenimine–magnetic iron oxide (PEG-PEI-IONPs) | microRNA-21 antisense oligonucleotides; Gemcitabine |
Female BALB/c nude mice | Pancreatic cancer | Reduction tumor growth; Inhibition of metastasis |
[95] |
| 31 | Gelatinases-stimuli poly(ethylene glycol)-peptide-poly(ε-caprolactone) copolymer | miR-200c Docetaxel |
BGC-823 gastric tumor-bearing mice | Gastric adenocarcinoma | Suppression tumor growth; Drug sensitivity; Inhibition of EMT |
[97] |
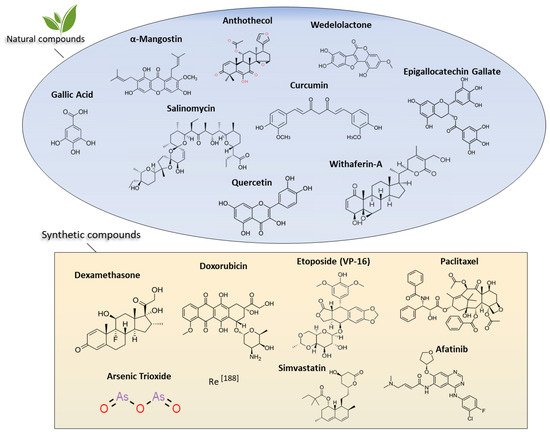
4.2.1. Natural Products
4.2.2. α-Mangostin and Anthothecol
4.2.3. Wedelolactone
4.2.4. Salinomycin
4.2.5. Gallic Acid
4.2.6. Curcumin
4.2.7. Quercetin
4.2.8. Epigallocatechin Gallate
4.3. Synthetic Drugs
4.3.1. Etoposide
4.3.2. ADH-1
4.3.3. Dexamethasone
4.3.4. Arsenic Trioxide
4.3.5. Rhenium-188
4.4. Nanoparticles for Nucleic Acids Delivery to Revert EMT Phenotype
4.4.1. Delivery of SiRNAs Against EMT TFs and Other EMT-Related Genes
4.4.2. SNAIL-1
4.4.3. ZEB1
4.4.4. TWIST1
4.4.5. Nogo-B Receptor
4.4.6. β3 Integrin
4.4.7. DCAMKL-1
4.4.8. Wnt-1
4.4.9. STAT3
4.4.10. AXL Kinase
4.5. Delivery of miRNAs Inhibiting EMT
4.5.1. miRNA-21
4.5.2. miRNA-139
4.5.3. miRNA-200c
4.5.4. Delivery of miRNAs Mimics
5. NP-Mediated Toxicology by Modulating EMT
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cancers12010025
References
- Nieto, M.A.; Huang, R.Y.Y.J.; Jackson, R.A.A.; Thiery, J.P.P. EMT: 2016. Cell 2016, 166, 21–45.
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476.
- Du, B.; Shim, J.S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 2016, 21, 965.
- Elaskalani, O.; Razak, N.B.A.; Falasca, M.; Metharom, P. Epithelial-mesenchymal transition as a therapeutic target for overcoming chemoresistance in pancreatic cancer. World J. Gastrointest. Oncol. 2017, 9, 37.
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488.
- DeVita, V.T.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653.
- Sinha, R. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917.
- Lu, Y.; Sun, W.; Gu, Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J. Control. Release 2014, 194, 1–19.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnol. 2018, 16, 71.
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowicz, C.; Cote, R.; Altman, N.; Datar, R.; et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016, 6, 20867.
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Aysıl Ozkan, S.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An effective tool in cancer therapy. Int. J. Pharm. 2018, 540, 132–149.
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339.
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134.
- Tan, E.J.; Olsson, A.K.; Moustakas, A. Reprogramming during epithelial to mesenchymal transition under the control of TGFβ. Cell Adhes. Migr. 2015, 9, 233–246.
- Strippoli, R.; Benedicto, I.; Lozano, M.L.P.; Cerezo, A.; López-Cabrera, M.; Del Pozo, M.A. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-κB/Snail1 pathway. Dis. Model. Mech. 2008, 1, 264–274.
- Stanisavljevic, J.; Porta-de-la-Riva, M.; Batlle, R.; de Herreros, A.G.; Baulida, J. The p65 subunit of NF-κB and PARP1 assist Snail1 in activating fibronectin transcription. J. Cell Sci. 2011, 124, 4161–4171.
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205.
- Bianchi, A.; Gervasi, M.E.; Bakin, A. Role of β5-integrin in epithelial-mesenchymal transition in response to TGF-β. Cell Cycle 2010, 9, 1647–1659.
- Strippoli, R.; Moreno-Vicente, R.; Battistelli, C.; Cicchini, C.; Noce, V.; Amicone, L.; Marchetti, A.; Del Pozo, M.A.; Tripodi, M. Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int. 2016, 2016, 3543678.
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601.
- Maurer, B.; Stanczyk, J.; Jüngel, A.; Akhmetshina, A.; Trenkmann, M.; Brock, M.; Kowal-Bielecka, O.; Gay, R.E.; Michel, B.A.; Distler, J.H.W.; et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010, 62, 1733–1743.
- Zheng, L.; Lin, S.; Lv, C. MiR-26a-5p regulates cardiac fibroblasts collagen expression by targeting ULK1. Sci. Rep. 2018, 8, 2104.
- Joo, D.; An, S.; Choi, B.G.; Kim, K.; Choi, Y.M.; Ahn, K.J.; An, I.S.; Cha, H.J. MicroRNA-378b regulates α-1-type 1 collagen expression via sirtuin 6 interference. Mol. Med. Rep. 2017, 16, 8520–8524.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890.
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736.
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest 2009, 119, 1420–1428.
- Spaderna, S.; Schmalhofer, O.; Wahlbuhl, M.; Dimmler, A.; Bauer, K.; Sultan, A.; Hlubek, F.; Jung, A.; Strand, D.; Eger, A.; et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008, 68, 537–544.
- Wellner, U.; Schubert, J.; Burk, U.C.; Schmalhofer, O.; Zhu, F.; Sonntag, A.; Waldvogel, B.; Vannier, C.; Darling, D.; Zur Hausen, A.; et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009, 11, 1487–1495.
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; Lebleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530.
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 1–12.
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16.
- Liu, Y.; Solomon, M.; Achilefu, S. Perspectives and potential applications of nanomedicine in breastand prostate cancer. Med. Res. Rev. 2013, 33, 3–32.
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316.
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157.
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44.
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chemie Int. Ed. 2014, 53, 12320–12364.
- Partha, R.; Lackey, M.; Hirsch, A.; Casscells, S.W.; Conyers, J.L. Self assembly of amphiphilic C60 fullerene derivatives into nanoscale supramolecular structures. J. Nanobiotechnology 2007, 5, 6.
- Partha, R.; Mitchell, L.R.; Lyon, J.L.; Joshi, P.P.; Conyers, J.L. Buckysomes: Fullerene-based nanocarriers for hydrophobic molecule delivery. ACS Nano 2008, 2, 1950–1958.
- Yang, W.; Thordarson, P.; Gooding, J.J.; Ringer, S.P.; Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007, 18, 412001.
- Williams, K.A.; Veenhuizen, P.T.M.; De la Torre, B.G.; Eritja, R.; Dekker, C. Nanotechnology: Carbon nanotubes with DNA recognition. Nature 2002, 420, 761.
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579.
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779.
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306.
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113.
- Oh, J.; Feldman, M.D.; Kim, J.; Condit, C.; Emelianov, S.; Milner, T.E. Detection of magnetic nanoparticles in tissue using magneto-motive ultrasound. Nanotechnology 2006, 17, 4183–4190.
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011, 11, 3744–3750.
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012, 6, 3767–3775.
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705.
- Xiong, X.; Arvizo, R.R.; Saha, S.; Robertson, D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget 2014, 5, 6453–6465.
- Huai, Y.; Zhang, Y.; Xiong, X.; Das, S.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 2019, 3, 267–279.
- Li, W.; Li, X.; Liu, S.; Yang, W.; Pan, F.; Yang, X.-Y.; Du, B.; Qin, L.; Pan, Y. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-—Mesenchymal transition inhibition. Int. J. Nanomed. 2017, 12, 3509–3520.
- Wu, Y.; Ali, M.R.K.; Dong, B.; Han, T.; Chen, K.; Chen, J.; Tang, Y.; Fang, N.; Wang, F.; El-Sayed, M.A. Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 2018, 12, 9279–9290.
- Kaushik, N.K.; Kaushik, N.; Yoo, K.C.; Uddin, N.; Kim, J.S.; Lee, S.J.; Choi, E.H. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials 2016, 87, 118–130.
- Li, X.; Song, L.; Hu, X.; Liu, C.; Shi, J.; Wang, H.; Zhan, L.; Song, H. Inhibition of epithelial-mesenchymal transition and tissue regeneration by waterborne titanium dioxide nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3449–3458.
- Peng, F.; Tee, J.K.; Setyawati, M.I.; Ding, X.; Yeo, H.L.A.; Tan, Y.L.; Leong, D.T.; Ho, H.K. Inorganic nanomaterials as highly efficient inhibitors of cellular hepatic fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 31938–31946.
- Wahab, R.; Kaushik, N.; Khan, F.; Kaushik, N.K.; Choi, E.H.; Musarrat, J.; Al-Khedhairy, A.A. Self-styled ZnO nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci. Rep. 2016, 6, 1–13.
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras G12D, and Kras G12D/tp53R270H) mice. Sci. Rep. 2016, 6, 1–13.
- Verma, R.K.; Yu, W.; Singh, S.P.; Shankar, S.; Srivastava, R.K. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2061–2070.
- Das, S.; Mukherjee, P.; Chatterjee, R.; Jamal, Z.; Chatterji, U. Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol. Cancer Ther. 2019, 18, 680–692.
- Daman, Z.; Faghihi, H.; Montazeri, H. Salinomycin nanoparticles interfere with tumor cell growth and the tumor microenvironment in an orthotopic model of pancreatic cancer. Drug Dev. Ind. Pharm. 2018, 44, 1434–1442.
- Sousa, C.; Gouveia, L.F.; Kreutzer, B.; Silva-Lima, B.; Maphasa, R.E.; Dube, A.; Videira, M. Polymeric micellar formulation enhances antimicrobial and anticancer properties of salinomycin. Pharm. Res. 2019, 36, 83.
- Sunil Gowda, S.N.; Rajasowmiya, S.; Vadivel, V.; Banu Devi, S.; Celestin Jerald, A.; Marimuthu, S.; Devipriya, N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. In Vitro 2018, 52, 170–177.
- Kumari, M.; Ray, L.; Purohit, M.P.; Patnaik, S.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur. J. Pharm. Biopharm. 2017, 117, 346–362.
- Kumari, M.; Purohit, M.P.; Patnaik, S.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loaded selenium nanoparticles synergize the anticancer potential of doxorubicin contained in self-assembled, cell receptor targeted nanoparticles. Eur. J. Pharm. Biopharm. 2018, 130, 185–199.
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697.
- Zheng, N.G.; Mo, S.J.; Li, J.P.; Wu, J.L. Anti-CSC effects in human esophageal squamous cell carcinomas and Eca109/9706 cells induced by nanoliposomal quercetin alone or combined with CD 133 antiserum. Asian Pac. J. Cancer Prev. 2014, 15, 8679–8684.
- Fan, J.X.; Zheng, D.W.; Rong, L.; Zhu, J.Y.; Hong, S.; Li, C.; Xu, Z.S.; Cheng, S.X.; Zhang, X.Z. Targeting epithelial-mesenchymal transition: Metal organic network nano-complexes for preventing tumor metastasis. Biomaterials 2017, 139, 116–126.
- Wang, Z.; Liang, P.; He, X.; Wu, B.; Liu, Q.; Xu, Z.; Wu, H.; Liu, Z.; Qian, Y.; Wang, S.; et al. Etoposide loaded layered double hydroxide nanoparticles reversing chemoresistance and eradicating human glioma stem cells in vitro and in vivo. Nanoscale 2018, 10, 13106–13121.
- Guo, Z.; Li, W.; Yuan, Y.; Zheng, K.; Tang, Y.; Ma, K.; Cui, C.; Wang, L.; He, B.; Zhang, Q. Improvement of chemosensitivity and inhibition of migration via targeting tumor epithelial-to-mesenchymal transition cells by ADH-1-modified liposomes. Drug Deliv. 2018, 25, 112–121.
- Li, W.; Guo, Z.; Zheng, K.; Ma, K.; Cui, C.; Wang, L.; Yuan, Y.; Tang, Y. Dual targeting mesoporous silica nanoparticles for inhibiting tumour cell invasion and metastasis. Int. J. Pharm. 2017, 534, 71–80.
- Guo, Z.; Zheng, K.; Tan, Z.; Liu, Y.; Zhao, Z.; Zhu, G.; Ma, K.; Cui, C.; Wang, L.; Kang, T. Overcoming drug resistance with functional mesoporous titanium dioxide nanoparticles combining targeting, drug delivery and photodynamic therapy. J. Mater. Chem. B 2018, 6, 7750–7759.
- Agarwalla, P.; Mukherjee, S.; Sreedhar, B.; Banerjee, R. Glucocorticoid receptor-mediated delivery of nano gold-withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine 2016, 11, 2529–2546.
- Huang, Y.; Zhou, B.; Luo, H.; Mao, J.; Huang, Y.; Zhang, K.; Mei, C.; Yan, Y.; Jin, H.; Gao, J.; et al. [email protected]2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling. Theranostics 2019, 9, 4391–4408.
- Lu, Y.; Liang, Y.; Zheng, X.; Deng, X.; Huang, W.; Zhang, G. EVI1 promotes epithelial-to-mesenchymal transition, cancer stem cell features and chemo-/radioresistance in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 1–17.
- Shen, Y.A.; Lan, K.L.; Chang, C.H.; Lin, L.T.; He, C.L.; Chen, P.H.; Lee, T.W.; Lee, Y.J.; Chuang, C.M. Intraperitoneal 188Re-Liposome delivery switches ovarian cancer metabolism from glycolysis to oxidative phosphorylation and effectively controls ovarian tumour growth in mice. Radiother. Oncol. 2016, 119, 282–290.
- Lin, L.T.; Chang, C.Y.; Chang, C.H.; Wang, H.E.; Chiou, S.H.; Liu, R.S.; Lee, T.W.; Lee, Y.J. Involvement of let-7 microRNA for the therapeutic effects of Rhenium-188-embedded liposomal nanoparticles on orthotopic human head and neck cancer model. Oncotarget 2016, 7, 65782–65796.
- Chang, C.-Y.; Chen, C.-C.; Lin, L.-T.; Chang, C.-H.; Chen, L.-C.; Wang, H.-E.; Lee, T.-W.; Lee, Y.-J. PEGylated liposome-encapsulated rhenium-188 radiopharmaceutical inhibits proliferation and epithelial-mesenchymal transition of human head and neck cancer cells in vivo with repeated therapy. Cell Death Discov. 2018, 4, 100.
- Jin, H.; He, Y.; Zhao, P.; Hu, Y.; Tao, J.; Chen, J.; Huang, Y. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 2019, 9, 265–278.
- Sadreddini, S.; Safaralizadeh, R.; Baradaran, B.; Aghebati-Maleki, L.; Hosseinpour-Feizi, M.A.; Shanehbandi, D.; Jadidi-Niaragh, F.; Sadreddini, S.; Kafil, H.S.; Younesi, V.; et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol. Lett. 2017, 181, 79–86.
- Afkham, A.; Aghebati-Maleki, L.; Siahmansouri, H.; Sadreddini, S.; Ahmadi, M.; Dolati, S.; Afkham, N.M.; Akbarzadeh, P.; Jadidi-Niaragh, F.; Younesi, V.; et al. Chitosan (CMD)-mediated co-delivery of SN38 and Snail-specific siRNA as a useful anticancer approach against prostate cancer. Pharm. Rep. 2018, 70, 418–425.
- Seifi-Najmi, M.; Hajivalili, M.; Safaralizadeh, R.; Sadreddini, S.; Esmaeili, S.; Razavi, R.; Ahmadi, M.; Mikaeili, H.; Baradaran, B.; Shams-Asenjan, K.; et al. SiRNA/DOX lodeded chitosan based nanoparticles: Development, characterization and in vitro evaluation on A549 lung cancer cell line. Cell. Mol. Biol. 2016, 62, 87–94.
- Fang, S.; Wu, L.; Li, M.; Yi, H.; Gao, G.; Sheng, Z.; Gong, P.; Ma, Y.; Cai, L. ZEB1 knockdown mediated using polypeptide cationic micelles inhibits metastasis and effects sensitization to a chemotherapeutic drug for cancer therapy. Nanoscale 2014, 6, 10084–10094.
- Shahin, S.A.; Wang, R.; Simargi, S.I.; Contreras, A.; Parra Echavarria, L.; Qu, L.; Wen, W.; Dellinger, T.; Unternaehrer, J.; Tamanoi, F.; et al. Hyaluronic acid conjugated nanoparticle delivery of siRNA against TWIST reduces tumor burden and enhances sensitivity to cisplatin in ovarian cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1381–1394.
- Roberts, C.M.; Shahin, S.A.; Wen, W.; Finlay, J.B.; Dong, J.; Wang, R.; Dellinger, T.H.; Zink, J.I.; Tamanoi, F.; Glackin, C.A. Nanoparticle delivery of siRNA against TWIST to reduce drug resistance and tumor growth in ovarian cancer models. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 965–976.
- Finlay, J.; Roberts, C.M.; Dong, J.; Zink, J.I.; Tamanoi, F.; Glackin, C.A. Mesoporous silica nanoparticle delivery of chemically modified siRNA against TWIST1 leads to reduced tumor burden. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1657–1666.
- Zhuo, X.; Chang, A.; Huang, C.; Yang, L.; Zhao, H.; Wu, Y.; Zhou, Q. Nanoparticle-mediated down-regulation of TWIST increases radiosensitivity of nasopharyngeal carcinoma cells via ERK pathway. Am. J. Cancer Res. 2015, 5, 1571–1579.
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed Res. Int. 2015, 2015, 382745.
- Wang, B.; Ding, Y.; Zhao, X.; Han, X.; Yang, N.; Zhang, Y.; Zhao, Y.; Zhao, X.; Taleb, M.; Miao, Q.R.; et al. Delivery of small interfering RNA against Nogo-B receptor via tumor-acidity responsive nanoparticles for tumor vessel normalization and metastasis suppression. Biomaterials 2018, 175, 110–122.
- Parvani, J.G.; Gujrati, M.D.; Mack, M.A.; Schiemann, W.P.; Lu, Z.R. Silencing β3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-negative breast cancer. Cancer Res. 2015, 75, 2316–2325.
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic lncRNA facilitates effective triple-negative breast cancer therapy. Bioconjug. Chem. 2019, 30, 907–919.
- Sureban, S.M.; May, R.; Mondalek, F.G.; Qu, D.; Ponnurangam, S.; Pantazis, P.; Anant, S.; Ramanujam, R.P.; Houchen, C.W. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnol. 2011, 9, 40.
- Ma, C.; Shi, L.; Huang, Y.; Shen, L.; Peng, H.; Zhu, X.; Zhou, G. Nanoparticle delivery of Wnt-1 siRNA enhances photodynamic therapy by inhibiting epithelial-mesenchymal transition for oral cancer. Biomater. Sci. 2017, 5, 494–501.
- Ma, Y.; Zhang, X.; Xu, X.; Shen, L.; Yao, Y.; Yang, Z.; Liu, P. STAT3 decoy oligodeoxynucleotides-loaded solid lipid nanoparticles induce cell death and inhibit invasion in ovarian cancer cells. PLoS ONE 2015, 10, e0124924.
- Suresh, D.; Zambre, A.; Mukherjee, S.; Ghoshdastidar, S.; Jiang, Y.; Joshi, T.; Upendran, A.; Kannan, R. Silencing AXL by covalent siRNA-gelatin-antibody nanoconjugate inactivates mTOR/EMT pathway and stimulates p53 for TKI sensitization in NSCLC. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102007.
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L.; et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503.
- Hong, S.-T.; Lin, H.; Wang, C.-S.; Chang, C.-H.; Lin, A.M.-Y.; Yang, J.C.-H.; Lo, Y.-L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 89.
- Liu, Q.; Li, R.T.; Qian, H.Q.; Wei, J.; Xie, L.; Shen, J.; Yang, M.; Qian, X.P.; Yu, L.X.; Jiang, X.Q.; et al. Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials 2013, 34, 7191–7203.
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327.
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881.
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763.
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400.
- Tang, Y.L.; Jiang, J.; Liu, J.; Zheng, M.; He, Y.W.; Chen, W.; Fan, Y.L.; Chen, Q.M.; Liao, C.H.; Liang, X.H. Hyperthermia inhibited the migration of tongue squamous cell carcinoma through TWIST2. J. Oral Pathol. Med. 2015, 44, 337–344.
- Jin, H.; Zhao, Y.; Zhang, S.; Yang, J.; Zhang, X.; Ma, S. Hyperthermia inhibits the motility of gemcitabine-resistant pancreatic cancer PANC-1 cells through the inhibition of epithelial-mesenchymal transition. Mol. Med. Rep. 2018, 17, 7274–7280.
- Kimura-Tsuchiya, R.; Ishikawa, T.; Kokura, S.; Mizushima, K.; Adachi, S.; Okajima, M.; Matsuyama, T.; Okayama, T.; Sakamoto, N.; Katada, K.; et al. The inhibitory effect of heat treatment against epithelial-mesenchymal transition (EMT) in human pancreatic adenocarcinoma cell lines. J. Clin. Biochem. Nutr. 2014, 55, 56–61.
- Yuan, G.-J. Hyperthermia inhibits hypoxia-induced epithelial-mesenchymal transition in HepG2 hepatocellular carcinoma cells. World J. Gastroenterol. 2012, 18, 4781.
- Xu, X.M.; Yuan, G.J.; Li, Q.W.; Shan, S.L.; Jiang, S. Hyperthermia inhibits transforming growth factor beta-induced epithelial-mesenchymal transition (EMT) in HepG2 hepatocellular carcinoma cells. Hepatogastroenterology 2012, 59, 2059–2063.
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228.
- Çeşmeli, S.; Biray Avci, C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766.
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.M.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid. Based Complement. Altern. Med. 2015, 2015, 593014.
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276.
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590.
- Tang, S.; Yin, Q.; Su, J.; Sun, H.; Meng, Q.; Chen, Y.; Chen, L.; Huang, Y.; Gu, W.; Xu, M.; et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly(β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials 2015, 48, 1–15.
- Hafeez, B.B.; Mustafa, A.; Fischer, J.W.; Singh, A.; Zhong, W.; Shekhani, M.O.; Meske, L.; Havighurst, T.; Kim, K.; Verma, A.K. α-mangostin: A dietary antioxidant derived from the pericarp of Garcinia mangostana L. inhibits pancreatic tumor growth in xenograft mouse model. Antioxid. Redox Signal. 2014, 21, 682–699.
- Jittiporn, K.; Suwanpradid, J.; Patel, C.; Rojas, M.; Thirawarapan, S.; Moongkarndi, P.; Suvitayavat, W.; Caldwell, R.B. Anti-angiogenic actions of the mangosteen polyphenolic xanthone derivative α-mangostin. Microvasc. Res. 2014, 93, 72–79.
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016, 48, 2155–2165.
- Shan, T.; Cui, X.; Li, W.; Lin, W.; Lu, H.; Li, Y.; Chen, X.; Wu, T. α-Mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacologica Sinica 2014, 35, 1065–1073.
- Lee, S.E.; Kim, M.R.; Kim, J.H.; Takeoka, G.R.; Kim, T.W.; Park, B.S. Antimalarial activity of anthothecol derived from Khaya anthotheca (Meliaceae). Phytomedicine 2008, 15, 533–535.
- Xu, Q.; Ma, J.; Lei, J.; Duan, W.; Sheng, L.; Chen, X.; Hu, A.; Wang, Z.; Wu, Z.; Wu, E.; et al. α-mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. Biomed Res. Int. 2014, 2014, 546353.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharm. 2018, 9, 1260.
- Sarveswaran, S.; Gautam, S.C.; Ghosh, J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt. Int. J. Oncol. 2012, 41, 2191–2199.
- Xu, D.; Lin, T.H.; Yeh, C.R.; Cheng, M.A.; Chen, L.M.; Chang, C.; Yeh, S. The wedelolactone derivative inhibits estrogen receptor-mediated breast, endometrial, and ovarian cancer cells growth. Biomed Res. Int. 2014, 2014.
- Yang, J.; Tao, L.; Liu, B.; You, X.; Zhang, C.; Xie, H.; Li, R. Wedelolactone attenuates pulmonary fibrosis partly through activating AMPK and regulating Raf-MAPKs signaling pathway. Front. Pharm. 2019, 10, 151.
- Schenk, M.; Aykut, B.; Teske, C.; Giese, N.A.; Weitz, J.; Welsch, T. Salinomycin inhibits growth of pancreatic cancer and cancer cell migration by disruption of actin stress fiber integrity. Cancer Lett. 2015, 358, 161–169.
- Zhang, G.N.; Liang, Y.; Zhou, L.J.; Chen, S.P.; Chen, G.; Zhang, T.P.; Kang, T.; Zhao, Y.P. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett. 2011, 313, 137–144.
- Mao, Z.; Wu, Y.; Zhou, J.; Xing, C. Salinomycin reduces epithelial–mesenchymal transition-mediated multidrug resistance by modifying long noncoding RNA HOTTIP expression in gastric cancer cells. Anti-cancer Drugs 2019, 30, 892–899.
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; Macmillan: London, UK; World Scientific: Singapore, 2009; pp. 239–250. ISBN 9789814287005.
- Paolini, A.; Curti, V.; Pasi, F.; Mazzini, G.; Nano, R.; Capelli, E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int. J. Oncol. 2015, 46, 1491–1497.
- Ho, H.-H.; Chang, C.-S.; Ho, W.-C.; Liao, S.-Y.; Wu, C.-H.; Wang, C.-J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516.
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171.
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Kim, H.S.; Kee, H.J.; Jeong, M.H. Gallic acid attenuates pulmonary fibrosis in a mouse model of transverse aortic contraction-induced heart failure. Vasc. Pharm. 2017, 99, 74–82.
- Cordani, M.; Somoza, Á. Targeting autophagy using metallic nanoparticles: A promising strategy for cancer treatment. Cell. Mol. Life Sci. 2019, 76, 1215–1242.
- Liu, Z.; Zhu, Y.Y.; Li, Z.Y.; Ning, S.Q. Evaluation of the efficacy of paclitaxel with curcumin combination in ovarian cancer cells. Oncol. Lett. 2016, 12, 3944–3948.
- Paramita, P.; Wardhani, B.W.K.; Wanandi, S.I.; Louisa, M. Curcumin for the prevention of epithelial-mesenchymal transition in endoxifen-treated MCF-7 breast cancer cells. Asian Pac. J. Cancer Prev. 2018, 19, 1243–1249.
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. Oncolytics 2016, 3, 16018.
- Zhao, J.L.; Guo, M.Z.; Zhu, J.J.; Zhang, T.; Min, D.Y. Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1). Cell. Mol. Biol. Lett. 2019, 24, 32.
- Yallapu, M.M.; Jaggi, M.C.; Chauhan, S. Curcumin nanomedicine: A road to cancer therapeutics. Curr. Pharm. Des. 2013, 19, 1994–2010.
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522.
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B Biointerfaces 2012, 94, 304–308.
- Zheng, S.; Li, X.; Zhang, Y.; Xie, Q.; Wong, Y.S.; Zheng, W.; Chen, T. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed. 2012, 7, 3939–3949.
- Liu, H.; Jiang, W.; Xie, M. Flavonoids: Recent advances as anticancer drugs. Recent Pat. Anti-cancer Drug Discov. 2010, 5, 152–164.
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial–mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial–mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. Oncol. Targets. Ther. 2017, 10, 4719–4729.
- Patel, D.; Sharma, N. Inhibitory effect of quercetin on epithelial to mesenchymal transition in SK-MEL-28 human melanoma cells defined by in vitro analysis on 3D collagen gels. Oncol. Targets. Ther. 2016, 9, 6445–6459.
- Hosta-Rigau, L.; Schattling, P.; Teo, B.M.; Lynge, M.E.; Städler, B. Recent progress of liposomes in nanomedicine. J. Mater. Chem. B 2014, 2, 6686–6691.
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed Res. Int. 2017, 2017, 5615647.
- Li, T.; Zhao, N.; Lu, J.; Zhu, Q.; Liu, X.; Hao, F.; Jiao, X. Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 2019, 10, 282–291.
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233.
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos. 2003, 31, 572–579.
- Huo, C.; Wan, S.B.; Lam, W.H.; Li, L.; Wang, Z.; Landis-Piwowar, K.R.; Chen, D.; Dou, Q.P.; Chan, T.H. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacology 2008, 16, 248–252.
- Gordaliza, M.; García, P.A.; Miguel Del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459.
- Sonabend, A.M.; Carminucci, A.S.; Amendolara, B.; Bansal, M.; Leung, R.; Lei, L.; Realubit, R.; Li, H.; Karan, C.; Yun, J.; et al. Convection-enhanced delivery of etoposide is effective against murine proneural glioblastoma. Neuro. Oncol. 2014, 16, 1210–1219.
- Needle, M.N.; Molloy, P.T.; Geyer, J.R.; Herman-Liu, A.; Belasco, J.B.; Goldwein, J.W.; Sutton, L.; Phillips, P.C. Phase II study of daily oral etoposide in children with recurrent brain tumors and other solid tumors. Med. Pediatr. Oncol. 1997, 29, 28–32.
- Eramo, A.; Ricci-Vitiani, L.; Zeuner, A.; Pallini, R.; Lotti, F.; Sette, G.; Pilozzi, E.; Larocca, L.M.; Peschle, C.; De Maria, R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006, 13, 1238–1241.
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67.
- Jin, F.; Zhao, L.; Guo, Y.-J.; Zhao, W.-J.; Zhang, H.; Wang, H.-T.; Shao, T.; Zhang, S.-L.; Wei, Y.-J.; Feng, J.; et al. Influence of etoposide on anti-apoptotic and multidrug resistance-associated protein genes in CD133 positive U251 glioblastoma stem-like cells. Brain Res. 2010, 1336, 103–111.
- Biasoli, D.; Kahn, S.A.; Cornélio, T.A.; Furtado, M.; Campanati, L.; Chneiweiss, H.; Moura-Neto, V.; Borges, H.L. Retinoblastoma protein regulates the crosstalk between autophagy and apoptosis, and favors glioblastoma resistance to etoposide. Cell Death Dis. 2013, 4, e767.
- Augustine, C.K.; Yoshimoto, Y.; Gupta, M.; Zipfel, P.A.; Selim, M.A.; Febbo, P.; Pendergast, A.M.; Peters, W.P.; Tyler, D.S. Targeting N-cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Cancer Res. 2008, 68, 3777–3784.
- Perotti, A.; Sessa, C.; Mancuso, A.; Noberasco, C.; Cresta, S.; Locatelli, A.; Carcangiu, M.L.; Passera, K.; Braghetti, A.; Scaramuzza, D.; et al. Clinical and pharmacological phase I evaluation of ExherinTM (ADH-1), a selective anti-N-cadherin peptide in patients with N-cadherin-expressing solid tumours. Ann. Oncol. 2009, 20, 741–745.
- Shintani, Y.; Fukumoto, Y.; Chaika, N.; Grandgenett, P.M.; Hollingsworth, M.A.; Wheelock, M.J.; Johnson, K.R. ADH-1 suppresses N-cadherin-dependent pancreatic cancer progression. Int. J. Cancer 2008, 122, 71–77.
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 2002, 110, 93–105.
- Zhang, L.; Lei, W.; Wang, X.; Tang, Y.; Song, J. Glucocorticoid induces mesenchymal-to-epithelial transition and inhibits TGF-β1-induced epithelial-to-mesenchymal transition and cell migration. FEBS Lett. 2010, 584, 4646–4654.
- Ferrand, N.; Stragier, E.; Redeuilh, G.; Sabbah, M. Glucocorticoids induce CCN5/WISP-2 expression and attenuate invasion in oestrogen receptor-negative human breast cancer cells. Biochem. J. 2012, 447, 71–79.
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13.
- Kim, J.H.; Hwang, Y.J.; Han, S.H.; Lee, Y.E.; Kim, S.; Kim, Y.J.; Cho, J.H.; Kwon, K.A.; Kim, J.H.; Kim, S.H. Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer. World J. Gastroenterol. 2015, 21, 9887–9899.
- Jang, Y.H.; Shin, H.S.; Sun Choi, H.; Ryu, E.S.; Jin Kim, M.; Ki Min, S.; Lee, J.H.; Kook Lee, H.; Kim, K.H.; Kang, D.H. Effects of dexamethasone on the TGF-β1-induced epithelial-to-mesenchymal transition in human peritoneal mesothelial cells. Lab. Investig. 2013, 93, 194–206.
- Bordag, N.; Klie, S.; Jürchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954.
- Wang, H.; Schaefer, T.; Konantz, M.; Braun, M.; Varga, Z.; Paczulla, A.M.; Reich, S.; Jacob, F.; Perner, S.; Moch, H.; et al. Prominent oncogenic roles of EVI1 in breast carcinoma. Cancer Res. 2017, 77, 2148–2160.
- Fan, Z.; He, J.; Fu, T.; Zhang, W.; Yang, G.; Qu, X.; Liu, R.; Lv, L.; Wang, J. Arsenic trioxide inhibits EMT in hepatocellular carcinoma by promoting lncRNA MEG3 via PKM2. Biochem. Biophys. Res. Commun. 2019, 513, 834–840.
- Kim, S.H.; Yoo, H.S.; Joo, M.K.; Kim, T.; Park, J.-J.; Lee, B.J.; Chun, H.J.; Lee, S.W.; Bak, Y.-T. Arsenic trioxide attenuates STAT-3 activity and epithelial-mesenchymal transition through induction of SHP-1 in gastric cancer cells. BMC Cancer 2018, 18, 150.
- Akhtar, A.; Wang, S.X.; Ghali, L.; Bell, C.; Wen, X. Recent advances in arsenic trioxide encapsulated nanoparticles as drug delivery agents to solid cancers. J. Biomed. Res. 2016, 31, 177–188.
- Argyrou, M.; Valassi, A.; Andreou, M.; Lyra, M. Rhenium-188 production in hospitals, by W-188/Re-188 generator, for easy use in radionuclide therapy. Int. J. Mol. Imaging 2013, 2013, 1–7.
- Zhang, H.; Tian, M.; Li, S.; Liu, J.; Tanada, S.; Endo, K. Rhenium-188-HEDP therapy for the palliation of pain due to osseous metastases in lung cancer patients. Cancer Biother. Radiopharm. 2003, 18, 719–726.
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169.
- Quijano, E.; Bahal, R.; Ricciardi, A.; Saltzman, W.M.; Glazer, P.M. Therapeutic peptide nucleic acids: Principles, limitations, and opportunities. Yale J. Biol. Med. 2017, 90, 583–598.
- Caplen, N.J.; Parrish, S.; Imani, F.; Fire, A.; Morgan, R.A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 2001, 98, 9742–9747.
- De Fougerolles, A.R. Delivery vehicles for small interfering RNA in vivo. Hum. Gene Ther. 2008, 19, 125–132.
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83.
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774.
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939.
- Khan, M.A.; Chen, H.C.; Zhang, D.; Fu, J. Twist: A molecular target in cancer therapeutics. Tumor Biol. 2013, 34, 2497–2506.
- Meng, Q.; Meng, J.; Ran, W.; Wang, J.; Zhai, Y.; Zhang, P.; Li, Y. Light-activated core–shell nanoparticles for spatiotemporally specific treatment of metastatic triple-negative breast cancer. ACS Nano 2018, 12, 2789–2802.
- Glackin, C.A. Nanoparticle delivery of TWIST small interfering RNA and anticancer drugs: A therapeutic approach for combating cancer. In Enzymes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 44, pp. 83–101. ISBN 9780128151112.
- Zhao, B.; Xu, B.; Hu, W.; Song, C.; Wang, F.; Liu, Z.; Ye, M.; Zou, H.; Miao, Q.R. Comprehensive proteome quantification reveals NgBR as a new regulator for epithelial–mesenchymal transition of breast tumor cells. J. Proteom. 2015, 112, 38–52.
- Wang, B.; Zhao, B.; North, P.; Kong, A.; Huang, J.; Miao, Q.R. Expression of NgBR is highly associated with estrogen receptor alpha and survivin in breast cancer. PLoS ONE 2013, 8, e78083.
- Wu, D.; Zhao, B.; Qi, X.; Peng, F.; Fu, H.; Chi, X.; Miao, Q.R.; Shao, S. Nogo-B receptor promotes epithelial–mesenchymal transition in non-small cell lung cancer cells through the Ras/ERK/Snail1 pathway. Cancer Lett. 2018, 418, 135–146.
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973.
- May, R.; Riehl, T.E.; Hunt, C.; Sureban, S.M.; Anant, S.; Houchen, C.W. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 2008, 26, 630–637.
- May, R.; Sureban, S.M.; Hoang, N.; Riehl, T.E.; Lightfoot, S.A.; Ramanujam, R.; Wyche, J.H.; Anant, S.; Houchen, C.W. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 2009, 27, 2571–2579.
- Sureban, S.M.; May, R.; Lightfoot, S.A.; Hoskins, A.B.; Lerner, M.; Brackett, D.J.; Postier, R.G.; Ramanujam, R.; Mohammed, A.; Rao, C.V.; et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011, 71, 2328–2338.
- Sureban, S.M.; May, R.; Ramalingam, S.; Subramaniam, D.; Natarajan, G.; Anant, S.; Houchen, C.W. Selective blockade of DCAMKL-1 results in tumor growth arrest by a let-7a microRNA-dependent mechanism. Gastroenterology 2009, 137, 649–659.e2.
- Prasad, C.P.; Mirza, S.; Sharma, G.; Prashad, R.; DattaGupta, S.; Rath, G.; Ralhan, R. Epigenetic alterations of CDH1 and APC genes: Relationship with activation of Wnt/β-catenin Pathway in invasive ductal carcinoma of breast. Life Sci. 2008, 83, 318–325.
- Saitoh, M.; Endo, K.; Furuya, S.; Minami, M.; Fukasawa, A.; Imamura, T.; Miyazawa, K. STAT3 integrates cooperative Ras and TGF-β signals that induce Snail expression. Oncogene 2016, 35, 1049–1057.
- Silver, D.L.; Naora, H.; Liu, J.; Cheng, W.; Montell, D.J. Activated signal transducer and activator of transcription (STAT) 3: Localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004, 64, 3550–3558.
- Nefedova, Y.; Gabrilovich, D. Targeting of Jak/STAT pathway in antigen presenting cells in cancer. Curr. Cancer Drug Targets 2007, 7, 71–77.
- Meyer, A.S.; Miller, M.A.; Gertler, F.B.; Lauffenburger, D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013, 6, ra66.
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013, 19, 279–290.
- Peng, Y.; Croce, C.M. The role of microRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004.
- Chen, Y.; Gao, D.-Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141.
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714.
- Li, Q.; Liang, X.; Wang, Y.; Meng, X.; Xu, Y.; Cai, S.; Wang, Z.; Liu, J.; Cai, G. MiR-139-5p inhibits the epithelial-mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by downregulating BCL2. Sci. Rep. 2016, 6, 27157.
- Liu, H.; Yin, Y.; Hu, Y.; Feng, Y.; Bian, Z.; Yao, S.; Li, M.; You, Q.; Huang, Z. -miR-139-5p sensitizes colorectal cancer cells to 5-fluorouracil by targeting NOTCH-1. Pathol. Res. Pract. 2016, 212, 643–649.
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443.
- Dykxhoorn, D.M. MicroRNAs and metastasis: Little RNAs go a long way. Cancer Res. 2010, 70, 6401–6406.
- Korpal, M.; Kang, Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008, 5, 115–119.
- Li, Y.; Vandenboom, T.G.; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009, 69, 6704–6712.
- Milán Rois, P.; Latorre, A.; Rodriguez Diaz, C.; del Moral, Á.; Somoza, Á. Reprogramming cells for synergistic combination therapy with nanotherapeutics against uveal melanoma. Biomimetics 2018, 3, 28.
- Pan, Y.; Zhang, J.; Fu, H.; Shen, L. miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. Oncol. Targets Ther. 2016, 9, 6247–6255.
- Imani, S.; Wei, C.; Cheng, J.; Khan, M.A.; Fu, S.; Yang, L.; Tania, M.; Zhang, X.; Xiao, X.; Zhang, X.; et al. MicroRNA-34a targets epithelial to mesenchymal transitioninducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget 2017, 8, 21362–21379.
- Choo, W.H.; Park, C.H.; Jung, S.E.; Moon, B.; Ahn, H.; Ryu, J.S.; Kim, K.S.; Lee, Y.H.; Yu, I.J.; Oh, S.M. Long-term exposures to low doses of silver nanoparticles enhanced in vitro malignant cell transformation in non-tumorigenic BEAS-2B cells. Toxicol. In Vitro 2016, 37, 41–49.
- Gliga, A.R.; Di Bucchianico, S.; Lindvall, J.; Fadeel, B.; Karlsson, H.L. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci. Rep. 2018, 8, 6668.
- Li, G.; Huang, Y.; Liu, Y.; Guo, L.; Zhou, Y.; Yang, K.; Chen, Y.; Zhao, G.; Lei, Y. In vitro toxicity of naturally occurring silica nanoparticles in C1 coal in bronchial epithelial cells. Zhongguo Fei Ai Za Zhi 2012, 15, 561–568.
- Guo, C.; You, D.Y.; Li, H.; Tuo, X.Y.; Liu, Z.J. Spherical silica nanoparticles promote malignant transformation of BEAS-2B cells by stromal cell-derived factor-1α (SDF-1α). J. Int. Med. Res. 2019, 47, 1264–1278.
- Ma, J.Y.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Scabilloni, J.; Ma, J.K.; Castranova, V. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol. Appl. Pharm. 2012, 262, 255–264.
- Park, E.J.; Cho, W.S.; Jeong, J.; Yi, J.H.; Choi, K.; Kim, Y.; Park, K. Induction of inflammatory responses in mice treated with cerium oxide nanoparticles by intratracheal instillation. J. Heal. Sci. 2010, 56, 387–396.
- Ma, J.; Bishoff, B.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Castranova, V. Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol. Appl. Pharm. 2017, 323, 16–25.
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul. Toxicol. Pharm. 2016, 74, 42–63.
- Wang, L.; Luanpitpong, S.; Castranova, V.; Tse, W.; Lu, Y.; Pongrakhananon, V.; Rojanasakul, Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011, 11, 2796–2803.
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338.
- Wang, P.; Voronkova, M.; Luanpitpong, S.; He, X.; Riedel, H.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Induction of slug by chronic exposure to single-walled carbon nanotubes promotes tumor formation and metastasis. Chem. Res. Toxicol. 2017, 30, 1396–1405.
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2015, 13, 27.
- Wang, P.; Wang, Y.; Nie, X.; Braïni, C.; Bai, R.; Chen, C. Multiwall carbon nanotubes directly promote fibroblast-myofibroblast and epithelial-mesenchymal transitions through the activation of the TGF-β/Smad signaling pathway. Small 2015, 11, 446–455.
- Lohcharoenkal, W.; Wang, L.; Stueckle, T.A.; Park, J.; Tse, W.; Dinu, C.Z.; Rojanasakul, Y. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front. Physiol. 2014, 5, 222.
- Chen, T.; Nie, H.; Gao, X.; Yang, J.; Pu, J.; Chen, Z.; Cui, X.; Wang, Y.; Wang, H.; Jia, G. Epithelial–mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol. Lett. 2014, 226, 150–162.
- Gui, S.; Li, B.; Zhao, X.; Sheng, L.; Hong, J.; Yu, X.; Sang, X.; Sun, Q.; Ze, Y.; Wang, L.; et al. Renal injury and Nrf2 modulation in mouse kidney following chronic exposure to TiO2 nanoparticles. J. Agric. Food Chem. 2013, 61, 8959–8968.
- Lee, S.B.; Kalluri, R. Mechanistic connection between inflammation and fibrosis. Kidney Int. 2010, 78, S22–S26.
- Hong, F.; Hong, J.; Wang, L.; Zhou, Y.; Liu, D.; Xu, B.; Yu, X.; Sheng, L. Chronic exposure to nanoparticulate TIO2 causes renal fibrosis involving activation of the Wnt pathway in mouse kidney. J. Agric. Food Chem. 2015, 63, 1639–1647.
- Wang, X.; Sun, B.; Liu, S.; Xia, T. Structure activity relationships of engineered nanomaterials in inducing NLRP3 inflammasome activation and chronic lung fibrosis. NanoImpact 2017, 6, 99–108.
- Yan, S.; Li, X.; Dai, J.; Wang, Y.; Wang, B.; Lu, Y.; Shi, J.; Huang, P.; Gong, J.; Yao, Y. Electrospinning of PVA/sericin nanofiber and the effect on epithelial-mesenchymal transition of A549 cells. Mater. Sci. Eng. C 2017, 79, 436–444.
- Li, X.; Yan, S.; Dai, J.; Lu, Y.; Wang, Y.; Sun, M.; Gong, J.; Yao, Y. Human lung epithelial cells A549 epithelial-mesenchymal transition induced by PVA/Collagen nanofiber. Colloids Surf. B Biointerfaces 2018, 162, 390–397.
