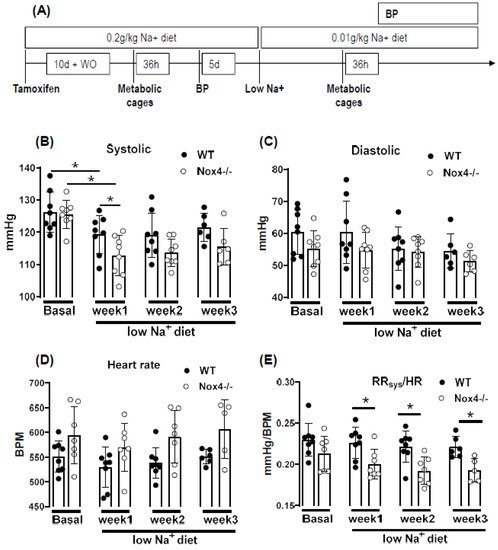
To study this aspect, mice were challenged with a low sodium diet: body weight, blood pressure and sodium excretion were first studied on regular chow (0.2 g/kg sodium), and subsequently on a low sodium diet (0.01 g/kg) applied for up to three weeks with prior tamoxifen-mediated knockout of Nox4 (Figure 2A).
Under normal chow, the blood pressure and heart rate of WT and Nox4-/- mice were similar. A reduction in sodium intake slightly lowered systolic blood pressure in both strains; this effect was more pronounced in Nox4-/- than in WT mice (only significant at week 1 of low Na+). There was also a trend towards a higher heart rate in Nox4-/- vs. WT mice (
Figure 2B–D). The values do not reach significance at all timepoints, potentially due to insufficient group size or high variability in tail cuff measurements. The combination of lower blood pressure and increased heart rate might indicate that peripheral resistance or plasma volume are different between WT and Nox4-/- mice. Indeed, whereas there was no difference under normal chow, when exposed to a low sodium diet, the ratio of systolic blood pressure to heart rate was significantly lower in Nox4-/- compared to WT mice (
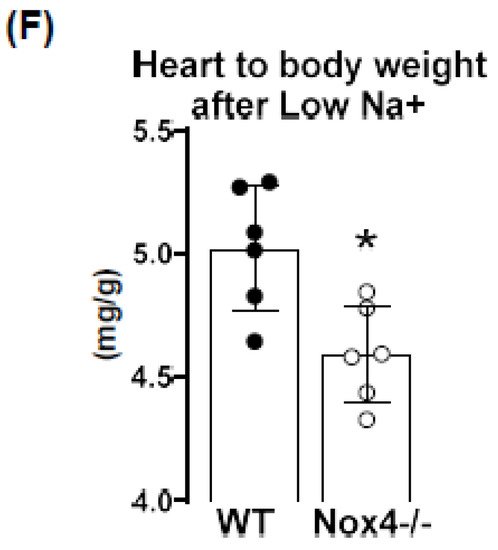
Figure 2E). Lower peripheral resistance or volume should attenuate cardiac load (after and preload, respectively), thus resulting in small hearts. Indeed, at the end of the 3-week diet, the heart to body weight ratio was lower in Nox4-/- as compared to WT mice, whereas the body weight was similar (
Figure 2F).
5. Discussion
In this study, we report that a low sodium diet results in an acute reduction in systolic blood pressure and a prolonged reduction in peripheral resistance in Nox4-/- mice. Despite the high expression of Nox4 in the kidney, this effect was unrelated to salt and water intake and renal sodium handling. Sodium restriction resulted in weight loss and renal sodium sparing, and this effect was similar between WT and Nox4-/- mice. Collectively, these data support the previous report of Nox4 in renal blood pressure control [
27] but exclude a direct effect on sodium handling as an underlying mechanism.
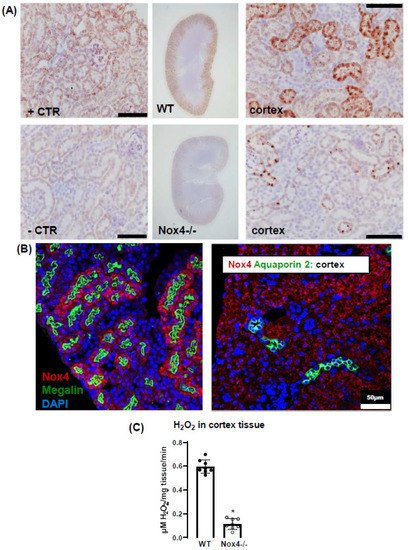
In the present study, we observe a high expression of Nox4 in the proximal renal tubule. Our data are in line with the single-cell sequencing atlas of the murine kidney [
28], where Nox4 is expressed in the cluster of proximal tubule cells and a novel cluster that is also positive for megalin. In addition, in the human kidney, Nox4 appears to localize to the proximal tubule [
18]. Despite these data on expression, very little is known concerning the function of Nox4 in the kidney. The location in the proximal tubule makes studying the function of Nox4 difficult, given that the physiological function of these cells depends on directionality, which is challenging in model systems of isolated cultured cells. Our past data indicated that Nox4 expression in the kidney is highest under quiescent conditions in healthy animals, whereas inflammation and diseases such as diabetes decreased Nox4 [
9]. The finding that renal disease reduces Nox4 expression has, meanwhile, been recapitulated by others [
18,
29] and suggests that Nox4 is a marker for healthy, differentiated, intact renal tissue. This behavior also has significance for renal cell culture models: the isolation of proximal tubule cells leads to a rapid loss in Nox4. Therefore, renal proximal tubule cell lines are alternatives. The opossum kidney OK cell line (from
Didelphis virginiana) is a broadly used model to study ion transport and membrane trafficking mechanisms in the proximal tubule. Transcriptomics of these cells, compared with mammalian proximal tubule cells, however, reveal that Nox4 expression is also lost in this cultured cell line [
30].
Therefore, the search for a function of Nox4 has to rely on in vivo data. Given that the transport processes in the proximal tubule are largely sodium-coupled, studying sodium handling and its indirect consequence, plasma volume and blood pressure, can be seen as first approaches to dissect the function of Nox4 in the kidney. The proximal tubule reabsorbs two-thirds of filtered Na+ [
31] and, consequently, is essential in sodium homeostasis. It is also important to note that, despite this behavior, the contribution of this renal segment to volume- and blood-pressure-control is, at least, controversial. From the knowledge available to date, the transport processes in this renal segment are not well controlled, potentially with the exception of phosphate reabsorption, which is inhibited by parathormone. Tubular-glomerular feedback (TGF), which controls proximal tubular urine flux, has a sensor in the distal tubule and affects glomerular filtration rate. To date, TGF has not been linked to Nox4; comparatively, it is associated with nitric oxide and adenosine [
32]. The second system that is relevant in conjunction with low sodium is the renin–angiotensin system (RAS) because a low sodium diet increases RAS activity by volume depletion and subsequent sympathetic nerve activation. The combination of increased sympathetic tone and RAS activation usually compensates for the effect of hypovolemia on blood pressure: cardiac output, renal water retention and peripheral resistance all increase. The fact that the blood pressure/heart rate ratio of Nox4 mice on a low sodium diet was significantly lower than that in wild-type mice, suggests that neither peripheral resistance nor volume retention can be adequately increased after deletion of Nox4. As body weight and sodium excretion were similar between WT and Nox4-/- mice, differences in peripheral resistance should be considered.
Interestingly, cerebral knockdown of Nox4 resulted in attenuated sympathico-excitation in response to cardiac damage [
33]. Whether Nox4 impacts directly on peripheral resistance is unclear. It has been suggested that SERCA is oxidized by Nox4 in the heart and endothelium [
34,
35] and also in TRP-channels [
36,
37,
38,
39], which contribute to the control of resting calcium, and are targets of Nox4. Moreover, Nox4 has been linked to smooth muscle cell differentiation and, thus, contractility [
40,
41,
42,
43]. Collectively, these observations provide support for a role of Nox4 in vascular tone control. Additional studies will be needed to substantiate this assumption.
Is there any evidence for a direct function of reactive oxygen species for renal hypertension? Superoxide anions lead to reduced medullary blood flow and increased sodium retention and, thus, hypertension [
7], as shown by in vivo treatment with a superoxide dismutase mimetic. Moreover, superoxide inhibits proximal tubule fluid reabsorption in spontaneously hypertensive rats [
44]. Renal hemodynamic and excretory functions, such as urine flow, sodium excretion and glomerular filtration were increased in hypertensive rats infused with the superoxide scavenger tempol without altering arterial pressure [
8]. In contrast, infusion of H
2O
2 directly into the renal medulla increases mean arterial pressure [
6]. Nox4 has been linked to renal hypertension and sodium retention in Dahl salt-sensitive rats, where volume expansion is considered to be the main cause of salt-sensitive hypertension [
45]. Our results corroborate the findings in the Dahl salt-sensitive rats; however, we cannot link the effect of Nox4 to Na
+ homeostasis because Na
+ excretion and clearance were similar in WT and Nox4-/- mice. On the other hand, the effects might have been so subtle and transient that our study was not sensitive enough to detect them.
The current study has several limitations. Blood pressure was measured by tail cuff technology, which is less accurate than telemetry. Moreover, we only estimated cardiac output and peripheral resistance from the blood pressure to heart rate ratio. A true determination of cardiac output and peripheral resistance would have required indicator injection dilution methodology. Moreover, metabolic cages impose a considerable amount of stress on mice. Food and fluid intake in the cages is low, which is also documented in the present study by the substantial weight loss. A possible alternative would have been clearance measurements using radioactive isotopes, but this technology was not available to us.