L-ascorbic acid, is a well-known molecule, sometimes used as antioxidant for skin care. Nonetheless, few studies have taken in account its utility as topical treatment for non-melanoma skin cancer. Non-melanoma skin cancer includes basal cell carcinoma and squamous cell carcinoma and is widespread worldwide with an increasing incidence.
- non-melanoma skin cancer (NMSC)
- basal cell carcinoma (BCC)
- squamous cell carcinoma (SCC)
- topical formulation
- skin cancer
- L-ascorbic acid (AA)
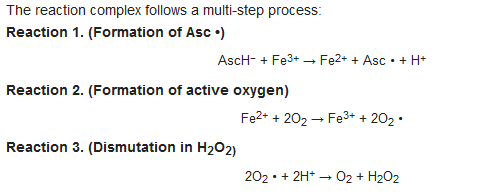
- ascorbyl radical (Asc •)
- hydrogen peroxide (H2O2)
- biodistribution
- pharmacokinetics
1. Introduction
2. L-Ascorbic Acid as a Pro-Drug
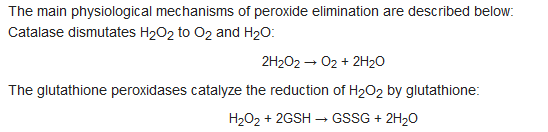
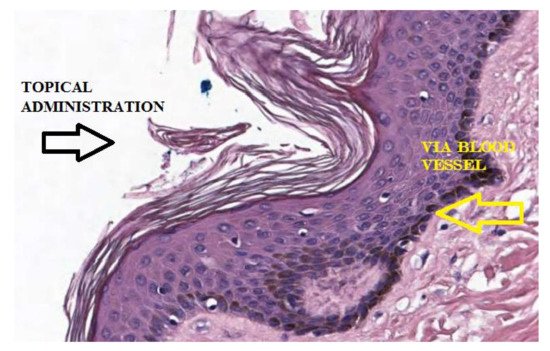
4. L-Ascorbic Acid in the Body
5. Specific Tissue Activation and AA Distribution
6. Physiological Role of AA in the Skin
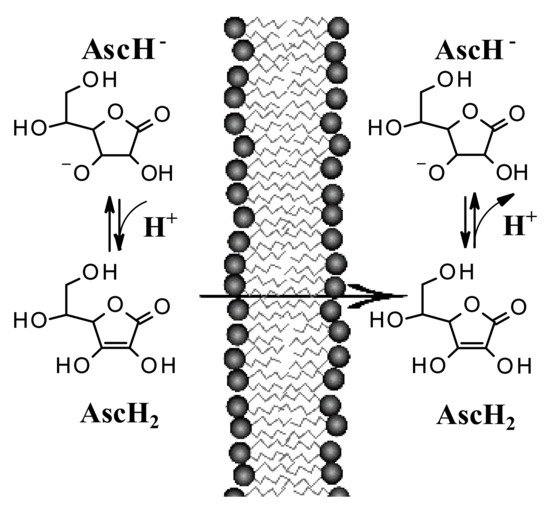
7. Passive Diffusion of AA and Implications for Its Topical Use
Recent studies suggest that encapsulation in a lipospheric form can promote transport into the lower layers of the epidermis and can ensure greater absorption. However, AA derivatives are not currently used in the field of skin cancers. Both its biodistribution through skin layers and through biological membranes has been little studied and the implications for its topical use are poorly understood. It has long been believed that this hydrophilic molecule only needs protein transporters to cross biological membrane barriers. The distribution of AA within the body requires bi-directional fluxes and, so far, only the AA transporters (SVCTs), facilitating its intake by cells have been identified. However, recent data suggest the implication of a possible passive transport of AA [18,19]. Experimental tests have shown that AA binds efficiently to the lipid bilayer interface and slowly crosses its hydrophobic core. It has been shown, using lipid membrane models, that AA crosses the lipid bilayer by passive diffusion and that the permeability coefficient depends on its protonation and high concentration gradients [19]. The permeability of the deprotonated AA (AscH−) has been determined to be orders of magnitude less than its protonated form (AscH2), so the AA must be protonated at the lipid bilayer interface before entering the hydrophobic region (Figure 4). It has been shown [18] that in AA homeostasis, the SVCT transporter is able to generate an AA concentration gradient across the plasma membrane by increasing its intracellular concentration. In turn, the concentration gradient generated by SVCT activity pushes the passive diffusion of AA out of the cell.
9. Etiology and Treatment of NMSC
Regarding the treatment of NMSC, certainly the complete surgical excision or RT, offers the highest and most reliable rates of disease control and a better prognosis.
In the treatment of BCC, in addition to radiotherapy were described further conservative treatments although with higher rates of persistence/ recurrence, that are photodynamic therapy, cryotherapy, curettage, electrodissection, use of intralesional alpha interferon, and the topical use of imiquimod. In the primary BCC for non-surgical treatments percentages of persistence or recurrence are reported: from 0% to 21% for cryotherapy; from 3% to 42% for curettage and electro-desiccation; from 2% to 30% for intralesional alpha interferon; 0% to 3% for photodynamic therapy; from 7% to 16% for radiotherapy; from 2% to 10% for surgical excision; from 7% to 21% for topical use of 5-fluorouracil; from 0% to 31% for topical use of imiquimod. In the literature, many studies certified the utility of the topical use of imiquimod and 5-fluorouracil fort the treatment of NMSC.
10. L-Ascorbic Acid for Topical Use in NMSC
The clinical cases [9,10,11] seem to confirm the rationale presented by Levine et al. [5] and Qi Chen et al. [6,7], according to which, AA would act as a pro-drug and how its pharmacokinetic plays a central role. These studies take into account the treatment of skin disease, considering the limitations related to biodistribution previously described and providing an empirical approach for topical treatment of BCC and SCC of the skin. It is therefore of interest to analyze how the chemical-physical characteristics of this formulation, can find confirmation in its clinical use. In this context, some considerations can be made. A topical formulation, with adequate concentrations of AA and pH values, can be prepared to obtain the best characteristics to ensure transport into the layers of the skin. AA in topical administration can generate Asc • and H2O2 directly in extracellular tissue, close to tumor cells, without implications related to its pharmacokinetics.
Asc • and H2O2 can be formed by site specific activation, mediated by metal proteins present in the tissues, bypassing the inhibitory action of the blood. Furthermore, continuous exposure over time to peroxide, capable of determining cytotoxicity towards tumor cells, would be more easily achievable than systemic administration.
AA is able to passively distribute itself across membranes [18,19] and therefore its transcutaneous absorption is directly influenced by the pH of the environment and the pKa of the molecule. It is known that the ionization of a molecule influences its diffusion, because cell membranes have a greater permeability towards its non-ionized form than the less fat-soluble ionized one. Generally, an acid vehicle improves the absorption of molecules with acid characteristics in the undissociated state and the concentration gradients determined by ion trapping can theoretically be very large if the difference in pH between the compartments considered is large. The difference in pH between the compartments plays a role in the absorption of drugs and in the case of acid topical formulations, the difference with the pH of the skin could determine a further contribution for its passage through the stratum corneum.
Activation of AA (as a pro-drug) should only occur in extracellular fluids and the formulation should not contain potentially interfering metal species to promote radical formation. The formulations used in the clinical cases considered were prepared extemporaneously [11] without excipients and with highly purified H2O. It can be hypothesized that the presence of potential metal catalysts can initiate the auto-oxidation of AA, favoring the formation of its radical before tissue activation.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13081201
