Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Gene therapy requires an effective and safe delivery vehicle for nucleic acids. Non-viral vehicles, including cationic liposomes, are intensively developed now. The structure of compounds composing them determines the delivery efficiency a lot. This review focuses on polycationic amphiphiles as prospective compounds for liposomal formulations and includes a discussion of the mutual influence of structural components.
- polyamines
- spermine
- cationic amphiphiles
- cationic liposomes
- gemini amphiphiles
- gene delivery
1. Introduction
Gene therapy is a modern and promising method for treating severe hereditary and acquired diseases, including COVID-19 immunization, through the delivery of therapeutic nucleic acids (NAs) that can replace a damaged gene (pDNA), provide a new one, or block the expression of an unwanted protein (antisense oligonucleotides, siRNA) [1][2]. The direct administration of therapeutic NAs is an inefficient process due to multiple external and internal limiting factors [3]. External factors lead to the instability of NAs in biological fluids (degradation by nucleases or interaction with albumin or low-density lipoproteins, causing the aggregation and rapid clearance of NAs) and a low degree of interaction with target cells. Internal factors are determined by the presence of membrane barriers (plasma, endosomal, and nuclear membranes) that present a challenge to NAs as they attempt to reach the cytosol and nucleus [4].
Overcoming these factors requires the development of special delivery vehicles. At present, viruses [5][6], which are highly effective but present some serious disadvantages, primarily associated with the induction of inflammatory and immune responses in the body, fill this role. However, alternative non-viral delivery vehicles, such as cationic liposomes (CLs) based on cationic amphiphiles (CAs) [6][7][8][9], are being developed. The most recent success is development of an mRNA vaccine [10] against COVID-19, where lipid nanoparticles deliver nucleoside-modified mRNA encoding a mutated form of the spike protein of SARS-CoV-2 [11]. Generally, CA structure is a combination of hydrophobic and cationic domains linked together by various spacer groups [12]. The positive charge of CAs enables the “packing” of NAs due to electrostatic interactions with the formation of lipoplexes (complexes of NAs with liposomes) and facilitates their interaction with a negatively charged plasma membrane.
In addition to CAs, liposomes can include helper lipids (for example, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DOPE) [13][14][15], which promote the formation of a certain lipid phase and favor cell transfection. Liposomes may also contain additional lipophilic molecules that permit them to target certain cells [16] or increase their circulation time in the bloodstream (for example, lipophilic derivatives of polyethylene glycol, PEG) [17]. If it is not stated below, CAs were used without additional components.
The CA structure significantly affects the efficiency of NA delivery to eukaryotic cells. In recent years, many monocationic amphiphiles have been obtained [18][19][20][21][22][23][24][25][26][27][28] that form liposomes for NA delivery. In this entry, we will consider polycationic amphiphiles, which, compared to monocationic analogs, enable the more efficient transport of NAs into cells due to the formation of a system of distributed charges in the polyamine matrix and their ability to facilitate NA release from endosomes. This ability is strongly affected by the high H+ buffer capacity of polyamines containing titratable amines results in endosomal Cl− accumulation during acidification with presumed osmotic endosome disruption and enhanced lipoplex escape [29].
2. Cationic Amphiphiles Based on Linear Polyamines
Enhancement of NA transport by polycationic amphiphiles may be related not only to distributed charges. On the cell surface, polyamine recognition sites—for example, PAT [30], a polyamine transporter—selectively transport both polyamines and their derivatives. Moreover, cancer cells have more such sites on their surfaces, which means that amphiphiles based on polyamines can transfect cancer cells more efficiently. Particularly important factors in NA delivery are the number and distribution of positive charges in the polyamine molecule. Transfection activity (TA) has been shown to increase as the number of amino groups in the polyamine structure increases. Compound 1e (Figure 1) exhibited the highest transfection efficiency among the synthesized lipopolyamines 1a–g [31], which suggests that 1e can use PAT and compete with other polyamines, for example, spermine, for binding to certain recognition sites on the cell surface (in particular, with the same PAT).
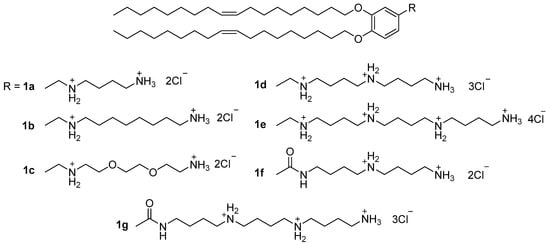
Figure 1. Lipopolyamines with benzyl linker.
Another factor affecting the efficiency of transfection is the hydrophobicity of the amphiphilic molecule. A study of compounds 2 and 3a–d, which contain sterols (cortisol and its derivatives) as hydrophobic domains (Figure 2), revealed that TA increases with an increase in the hydrophobicity of the molecule [32]. While liposomes with compound 3d were shown to be incapable of delivering NAs, possibly due to the lower hydrophobicity of the amphiphile 3d and ineffective formation of lipoplexes, compounds 3b and 3c had the highest transfection efficiencies. Notably, the contribution of hydrophobicity to the efficiency of NA delivery also depends on other parameters, primarily the CA/DOPE (the last was a helper lipid) ratio and N/P ratio (the ratio of the number of CA amino groups to the number of NA phosphate groups).
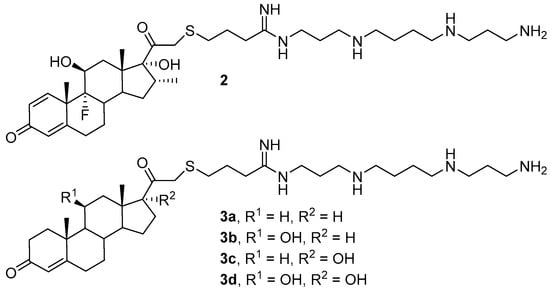
Figure 2. Cationic amphiphiles (CAs) based on cortisol and its derivatives.
Subsequent studies have shown [33] that compounds 4b and 4c, which contain double bonds in the polycyclic hydrophobic domain (Figure 3), were the most effective. Three-component liposomes formed from compound 4d, its dimeric analog 4e, and DOPE delivered plasmid DNA (pDNA) more efficiently than two-component liposomes 4e/DOPE.
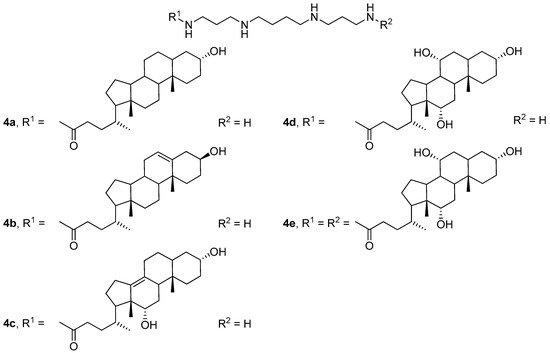
Figure 3. CAs with different polycyclic hydrophobic domains.
Hydrophobic substituents in the CA structure are mainly attached to primary amino groups of the polyamine. A different approach to the synthesis of CAs was proposed by Blagbrough et al. [34][35][36][37], who obtained N4,N9-disubstituted spermine derivatives 5a–j with acyl or alkyl residues of various lengths and degrees of unsaturation (Figure 4). All lipoplexes formed from acyl-substituted polyamines 5a–h exhibited high TA, excluding amphiphiles 5b and 5f. However, only compounds 5f and 5g with stearoyl and oleoyl residues had low toxicity toward FEK4 and HtTA cells. Notably, an increase in the degree of unsaturation of hydrocarbon chains increased both the efficiency of transfection and the cytotoxicity of the compounds. Alkyl derivatives of spermine 5i and 5j had comparable or slightly higher transfection efficiencies but were much more toxic than their acyl analogs [35].
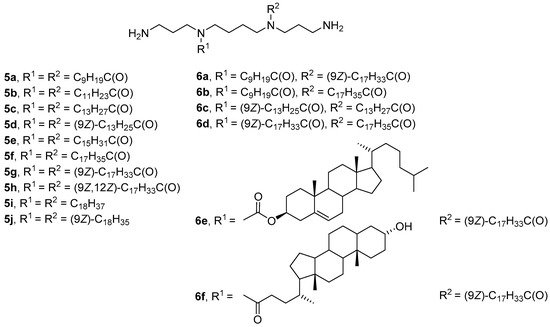
Figure 4. N4,N9-disubstituted spermine derivatives.
Asymmetric analogs 6a–f (Figure 4) were subsequently developed [38]. N4-myristoleoyl-N9-myristoylspermine (6c) and N4-oleoyl-N9-stearoylspermine (6d) showed the highest efficiency of siRNA delivery into FEK4 and HtTA cells, comparable to the efficiency of the commercial transfectant TransIT-TKO (Mirus Bio, Madison, WI, USA). Amphiphile 6f with a lithocholoyl residue effectively delivered NAs but caused cell death. The least effective was N4-cholesteryl-N9-oleoylspermine (6e).
When N1,N12-substituted spermine derivatives 7a–d (Figure 5), structural isomers of amphiphiles 5c, 5d, 5f, 5g, were synthesized and studied [39], the efficiency of pDNA delivery into FEK4 and HtTA cells by complexes with amphiphiles 7a–d was lower, while the toxicity was higher than with amphiphile 5g. siRNA delivery efficiency mediated by compounds 7a and 7c was comparable to the efficiency of delivery using amphiphile 5g.
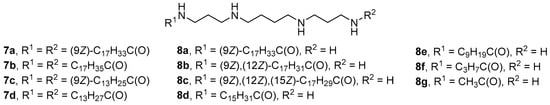
Figure 5. Spermine derivatives with acyl-substituted terminal amino groups.
Multiple monosubstituted polyamine derivatives 8a–g (Figure 5) were obtained by modifying spermine with fatty acid residues of various lengths and degrees of unsaturation [40]. Although an increase in the length of the fatty acid residue increased toxicity, it positively affected the penetration of lipoplexes through the cell membrane in vitro. Experiments in vivo showed that the efficiency of NA delivery with N-butanoylspermine (8f) was higher than with N-decanoylspermine (8e).
Mono- and disubstituted polycationic amphiphiles were developed based on spermine as a hydrophilic domain and cholesterol or 1,2-di-O-tetradecylglycerol as hydrophobic domains (Figure 6) [41][42][43]. The amphiphiles had different spacer lengths and linker types. CLs were prepared using these amphiphiles and DOPE (1:1 mol.). Among monosubstituted amphiphiles 9a–c, compound 9b showed the highest TA. While transfecting the same percentage of cells as their monomeric analogs 9a–c, however, dimeric polycationic amphiphiles 10a–c provided better expression of the green fluorescent protein. The highest transfection efficiency was exhibited by liposomes based on amphiphile 10c, which were superior to the efficiency of the commercial transfectant Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) for any type of NAs transferred [42][43]. Targeted liposomes based on CA 10c were also successfully employed in vivo [44][45][46][47][48][49].
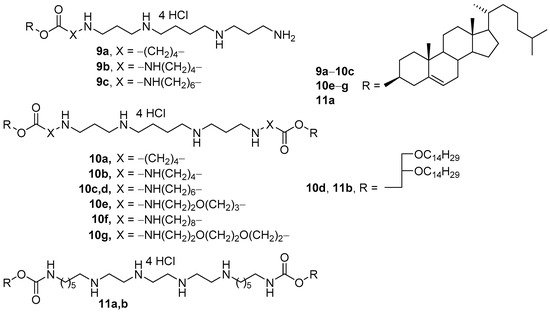
Figure 6. Mono- and dimeric polycationic amphiphiles based on spermine and triethylenetetramine.
Analogs 10e–g with ethoxypropylene, octamethylene, and ethoxyethoxyethylene spacers (Figure 6) permitted a greater TA increase than using 10c in vitro [50]. In contrast, the replacement of spermine with triethylenetetramine (TETA, 11a,b) led to a significant decrease in TA [51].
Extensive screening of CAs [52] revealed that in the structure of compounds 12a–j through 29a–j, both the polyamine matrix and the hydrophobic components changed (Figure 7). Among CLs formed from these amphiphiles and DOPE (1:2 weight ratio), the effective transfection of HEK293 cells was achieved only by liposomes with amphiphiles 12a–j through 20a–j containing an acyl substituent at the terminal amino group. Moreover, only eight compounds (12c, 12e, 13d, 14c, 16d, 16g, 17h, and 17j) were superior in TA to the commercial transfectant Effectene (Qiagen, Hilton, Germany). Subsequent transfection studies on HEK293, COLO 205, D17, HeLa, and PC3 cells showed that these compounds mediated more effective NA transport than did the commercial transfectants Effectene, DOTAP, and DC-Chol, while their toxicity was lower than that of commercial transfectants.
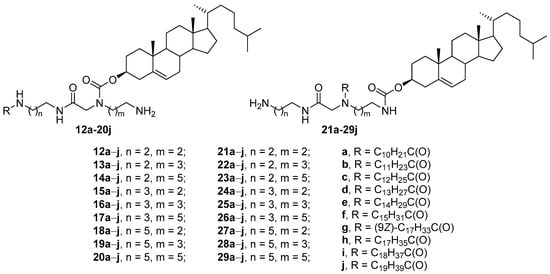
Figure 7. CAs with asymmetric acyl hydrophobic tails.
pH-Sensitive polycationic amphiphiles 30–33 (Figure 8) were obtained by subsequently coupling amino acids (l-histidine and l-cysteine) and fatty acids (lauric, oleic, and stearic) to polyamines [53][54]. The size of complexes of amphiphiles with siRNA was 160–210 nm, and the maximum TA on U87 cells was achieved using amphiphiles 30b–33b with oleic residues. Among them, TA decreased in the series 30b > 32b > 31b > 33b. A correlation was also established between the TA and the ability of compounds 30b–33b to disrupt the integrity of erythrocyte membranes. Leader compound 30b based on ethylenediamine exhibited the highest hemolytic activity at pH 5.4, which corresponds to the onset of endosomal acidification. Therefore, when using this amphiphile, one can expect effective NA release inside cells due to the disruption of endosomal membranes.
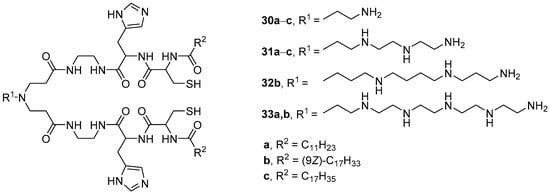
Figure 8. First generation of pH-sensitive polycationic amphiphiles.
The second generation of pH-sensitive amphiphiles 34a–h based on spermine was subsequently obtained (Figure 9). In biological tests conducted on HeLa and U87 cells, the presence of an l-histidine in the amphiphile structure did not improve TA. In addition, no relationship was found between the efficiency of CAs and the distance between hydrophobic domains. Compound 34e exhibited the highest activity in the delivery of pDNA [55], while amphiphile 34f exhibited the highest activity in the delivery of siRNA [56][57]. The authors also noted that they did not utilize helper lipids in complex formation since the synthesized compounds were able to initiate a pH-dependent phase transition, which led to the destabilization of the complexes and the release of NAs.
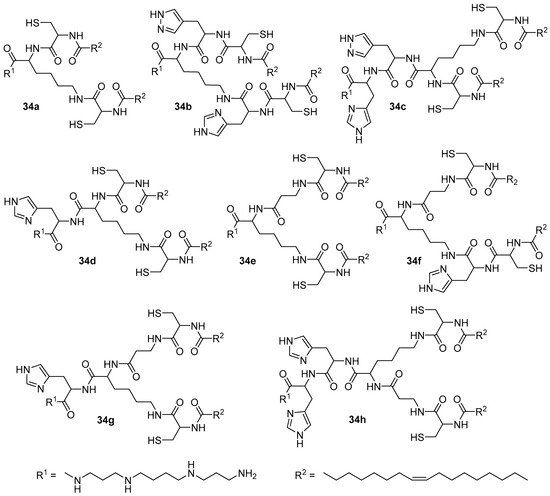
Figure 9. Second generation of pH-sensitive polycationic amphiphiles.
Multiple phosphamide derivatives containing long-chain alkyl substituents (dodecyl, tetradecyl, and hexadecyl) were obtained as hydrophobic fragments [58]. The transfection of COS-1 cells with lipoplexes formed by pDNA and micelles or liposomes based on amphiphiles 35a–d (Figure 10) showed that complexes based on micelles were only half as effective as complexes based on CA liposomes/lipid helper/Chol (1:1:1 mol.). DOPE and dipalmitoyl phosphatidylcholine (DPPC) have been used as helper lipids, but DPPC-containing liposomes have proven to be an ineffective delivery vehicle. TA on LLC and B16BL6 cells increased with an increase in the length of the alkyl chains and the number of amino groups in the polyamine. For LLC cells, the best compound was 35f based on spermine, and for B16BL6 cells, the best compound was amphiphile 35c based on spermidine.
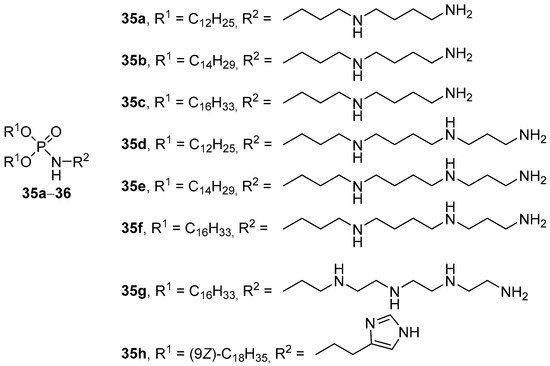
Figure 10. Phosphamide derivatives of polyamines.
An analog of compounds 35c and 35f was obtained based on a synthetic polyamine–tetraethylenepentamine (35g, Figure 10) [59]. Liposomes 35g/DOPE/DPPC/Chol (0.25:1:0.75:1 mol) efficiently delivered antisense oligonucleotides to eukaryotic cells. Here, the introduction of lipophilic derivatives of polyethylene glycol (PEG) and a cyclic analog of the peptide RGD ensured active targeting of liposomes to target cells and increased the efficiency of NA delivery [60][61].
Cationic nucleoside amphiphiles may also be used for gene delivery. Thus, low-toxic uridine derivatives of various polyamines (36a–c, Figure 11) were synthesized and used for siRNA delivery. Their TA on HeLa cells is almost equal to that of Lipofectamine 2000 but was not affected by polyamine residue [62]. Notably, replacement of polyamine residue with L-arginine gave the same results, while l-lysine decreased TA [63].
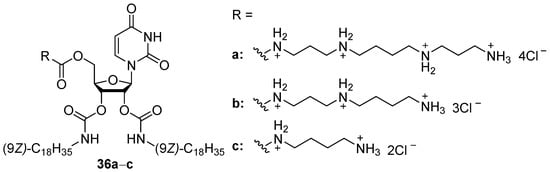
Figure 11. Cationic nucleoside amphiphiles.
Amphiphiles 37a–d (Figure 12), in which the polyamine was bound to the hydrophobic domain via carbamoyl or amide linkers, formed liposomes with DOPE or compound 35h (Figure 11) and were used to deliver pDNA [64]. Protonation of the imidazolium residue of amphiphile 35h during endosomal acidification can induce rupture of the endosomal membrane and favors NA release [65][66]. Transfection of OVCAR-3, IGROV-1, and HeLa cells with complexes formed at different N/P ratios (4:1–12:1) showed that 37c/DOPE liposomes provided efficient pDNA delivery exceeding that of the commercial transfectant Lipofectamine 2000. Relative TA decreased in the series 37c > 37b > 37a >> 37d. It should also be noted that the use of amphiphile 36 as a helper lipid did not increase TA but did increase the cytotoxicity of lipoplexes.
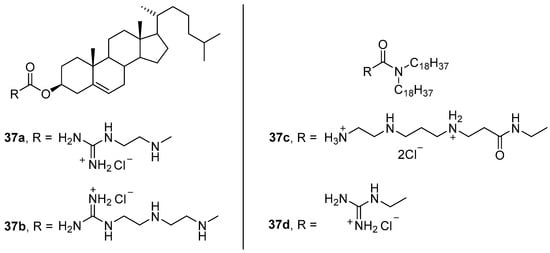
Figure 12. Amphiphiles based on polyamines and cholesterol.
In vivo delivery of pDNA by sterically stabilized liposomes 37c/DOPE/PEG4600-Chol (43:43:14 mol.) in a 4:1 (wt) ratio with pDNA led to a 33-fold increase in protein expression relative to unprotected DNA [67].
New CAs 38a–c (Figure 13), in which the cationic domain was linked to the cholesterol residue via an ether bond [68], formed liposomes with DOPE and were used for transfection of AGS and Huh-7 cells. The 38a/DOPE liposomes more efficiently delivered pDNA into AGS cells, while the 38b/DOPE liposomes provided effective transfection of Huh-7 cells. In both cases, their TA exceeded that of commercial transfectants [69]. Liposomes with dimeric gemini-amphiphile 38c also outperformed commercial agents in the transfection of COS-7 and Huh-7 cells [70].
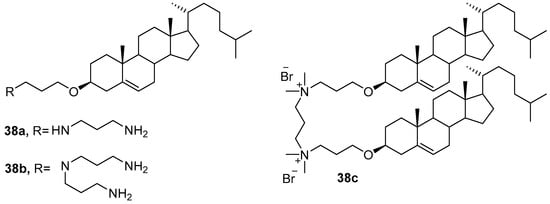
Figure 13. Ether-linked cationic amphiphiles.
CAs 39a,b with different dicationic domains (Figure 14) formed liposomes, which facilitated the transport of siRNA into MB49 and K562 cells, while amphiphile 39a was superior in TA to amphiphile 39b [71].
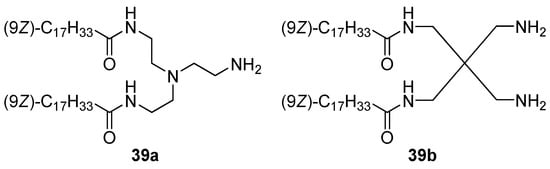
Figure 14. Branched cationic amphiphiles.
Comparing the TA of CAs that contained various polyamines in their structure (Figure 15) revealed that CLs composed of both phosphatidylcholine (Phospholipon 90G) and compounds 40d–g containing spermine (5:1 mol.) could deliver pDNA to HeLa cells, while other CLs showed no transfection [72]. Moreover, CAs with a shorter chain length of acyl substituents exhibited lower TA.
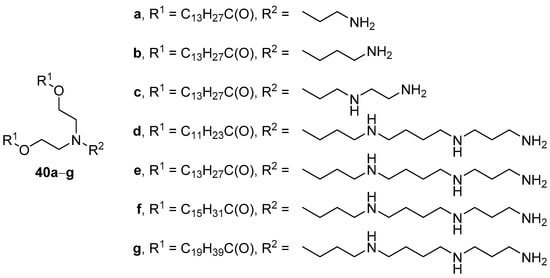
Figure 15. CAs based on various polyamines.
Analogs of compounds 40d–f based on spermine (compounds 41a–c, 42a–c, 43a–c) were obtained to study the influence of the structure core on TA (Figure 16) [73]. The efficiency of pDNA delivery mediated by liposomes based on DOPE and amphiphiles 42b,c, and 43a (1:1, weight ratio) was higher or comparable to that of Lipofectamine 2000. Moreover, unlike the other formulations, liposomes based on 43a with a core of 2-amino-1,3-propanediol retained their efficiency in the presence of serum. Investigation of the effect of hydrophobic domains on transfection revealed that myristoyl residues provided more effective TA.
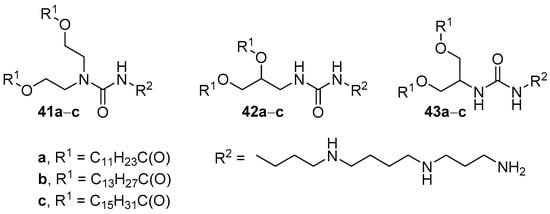
Figure 16. Spermine-based CAs with different cores.
A library of CAs (more than 1200 compounds) was developed using combinatorial chemistry methodology, in which both the hydrophobic (the length of the alkyl chain, the type of linker, and the presence of additional functional groups) and the cationic (the number of amino groups, the presence of cycles, and other functional groups) domains varied [74]. The results of in vitro experiments on HeLa cells revealed the following relationships: (1) TA increased in the presence of either two long-chain or several shorter alkyl substituents linked by an amide bond to the cationic domain (the optimal length was 8–12 carbon atoms); (2) high TA was achieved by compounds with two or more amino groups in the cationic domain, with TETA offering the best option; (3) the presence of a secondary amino group in the cationic domain positively affected TA (Figure 17).
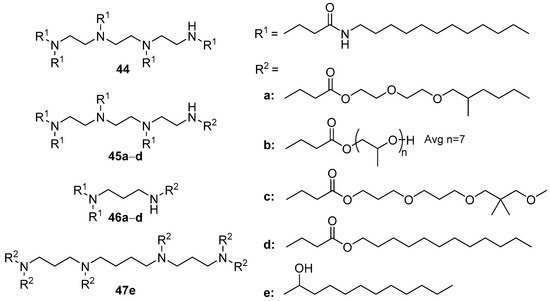
Figure 17. A combinatorial library of CAs.
Based on these findings, multiple CAs were selected for extended biological studies, which showed that the efficiency of NA delivery to primary macrophages exceeded that of commercial transfectants. In contrast, the transfection of HeLa or HepG2 cells by the selected amphiphiles was poor.
According to the results of in vitro tests, the 17 most effective compounds for in vivo siRNA delivery (siFVII and siApoB, which suppress the expression of blood coagulation factor VII and apolipoprotein B, respectively), were selected. For this, liposomes containing CA/PEG-lipid (mPEG2000-palmitoylceramide or mPEG2000-dimyristoylglycerol)/Chol (42:10:48 mol.) were formed. These CAs commonly contained various diamines or TETA. The most effective formulation (achieving more than 90% suppression of target gene expression) was based on compound 44 with TETA (Figure 17). The delivery of siRNAs in the lungs and macrophages of mice and macaque liver cells using these liposomes also significantly suppressed the target genes [74].
Subsequently, the library of compounds was expanded by synthesizing amphiphiles 45а–d and 46а–d with various hydrophobic domains (TETA and propylenediamine were chosen as cationic domains). Of these, the most effective were amphiphiles with a hydroxyl group in the hydrophobic domain, while the domain itself was bound to the polyamine with an ether or amide linker [75]. The hydrophobic domains based on oligoethylene glycol or octadecyl substituents led to an absence of TA. It should be noted that CAs 45c, 46a, and 46d were more effective than amphiphile 44 in vitro; however, they were inferior to amphiphile 44 in siRNA delivery in vivo.
In vitro screening of amphiphilic derivatives of spermine, spermidine, putrescine, and cadaverine showed that spermine derivative 47e facilitated the more efficient delivery of siRNA into human hepatocellular carcinoma Huh-7 cells [76]. Furthermore, liposomes 47e/Chol/DSPC/mPEG2000-palmitoylceramide/galactosylceramide delivered siRNA in vivo, which led to a significant decrease in hepatitis C virus replication in the hepatocyte cells of mice [76].
3. Influence of Structural Components of Cationic Amphiphiles on the Efficiency of Nucleic Acid Delivery
Each element of the CA structure performs a specific function and influences the TA. Hydrophobic domains are involved in the protection of NAs and promote the fusion of lipoplexes with cell membranes. Aliphatic hydrocarbon substituents usually represent these domains with a length of 10 to 18 carbon atoms, tocopherol, or sterols. The type of hydrophobic domain determines both the structure of the vesicles that a CA forms in the aqueous phase and its subsequent interaction with biological membranes. Liposomal formulations promote more efficient NA delivery than that accomplished by micelles or other types of nanoparticles [58]. Mostly, CAs used for transfection of eukaryotic cells are classic head-to-tail amphiphiles. Based on polyamines and amino acids, CAs synthesized with two hydrophobic domains (gemini-amphiphiles) can deliver NAs more efficiently than can their monosubstituted analogs [[39][42].
An increase in the length of aliphatic hydrocarbon chains usually increases TA [32,35,40,53,58]. Notably, however, it may also increase the toxicity of compounds [40]. In contrast, for some spermine-based CAs, TA decreased with an increase in the length of aliphatic substituents [70]. Analysis of published data reveals that the
optimal length of aliphatic substituents is 14–18 carbon atoms, while high TA is most often noted for CAs with myristoyl or tetradecyl substituents [71]. The degree of unsaturation of substituents also affects the efficiency of NA delivery: with an increase in unsaturation, TA increases but so does toxicity [20]. Thus, it is necessary to search for an optimal CA variant that effectively delivers NAs, while its toxicity remains within acceptable limits.
optimal length of aliphatic substituents is 14–18 carbon atoms, while high TA is most often noted for CAs with myristoyl or tetradecyl substituents [71]. The degree of unsaturation of substituents also affects the efficiency of NA delivery: with an increase in unsaturation, TA increases but so does toxicity [20]. Thus, it is necessary to search for an optimal CA variant that effectively delivers NAs, while its toxicity remains within acceptable limits.
When sterol derivatives are used as hydrophobic domains, one should prefer natural compounds, which do not cause significant toxicity. In this case, it is optimal to use a common and widely available sterol such as cholesterol [38], although diosgenin derivatives are also capable of efficient NA delivery [75][76].
The positively charged CA domain is responsible for the electrostatic interactions of amphiphiles and/or liposomes with NAs, the formation of stable lipoplexes, and the interaction of complexes with cell membranes. An increase in the number of amino groups in the structure of the cationic domain leads to an increase in the TA [31,56,60]. The most effective are CAs with domains based on polyamines, with the number (more than two [74]) and distribution of amino groups in the polyamine chain [31,53] playing important roles. Many studies reveal that the most effective are polyamines with four amino groups, primarily a natural polyamine—spermine [58][72][76]. However, CAs based on synthetic polyamines (TETA, triethylenepentamine, tributylenepentamine) can be more efficient in delivering NAs than their natural counterparts [31][59][74]].
Linkers, connecting hydrophobic and cationic domains, determine the stability and biocompatibility of the CAs and play a key role in the efficiency of NA delivery. The most commonly used are ether, ester, carbamate, amide, and disulfide linkers. Among the most effective linkers imparting low toxicity to CAs are carbamoyl ones [42][43][52]. Efficient delivery of NAs is also observed with the presence of ether bonds in the CA structure [68].
Multiple studies have proven that a close arrangement of the hydrophobic and cationic domains complicates both domain’s functioning and interferes with the formation of liposomes. The introduction of a spacer into the CA structure and an increase in its length increase NA delivery efficiency [42][50].
Low cytotoxicity is an important feature of CLs. First generation of CAs containing quaternary ammonium head was rather toxic, but numerous recently developed polycationic amphiphiles provided non-toxic transfection [8]. As mentioned above, toxicity may be increased with the length and degree of unsaturation of hydrophobic tails. Also, it should be noted that direct binding of both hydrophobic and cationic domains leads to a significant increase in toxicity of the CAs [35].
In conclusion, the development of effective and safe CLs for the delivery of therapeutic NAs requires employment of the right combination of structural elements in the CA molecule to promote the formation of both the liposomes and their complexes with NAs while avoiding interference and overcoming biological barriers. Once within the target cell, the complexes must release NAs with a high efficiency to provide biological/therapeutic effect.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13060920
References
- Behr, J.P. Synthetic gene transfer vectors II: Back to the future. Acc. Chem. Res. 2012, 45, 980–984.
- Räty, J.K.; Pikkarainen, J.T.; Wirth, T.; Ylä-Herttuala, S. Gene therapy: The first approved gene-based medicines, molecular mechanisms and clinical indications. Curr. Mol. Pharmacol. 2008, 1, 13–23.
- Wiethoff, C.M.; Middaugh, C.R. Barriers to nonviral gene delivery. J. Pharm. Sci. 2003, 92, 203–217.
- Gottfried, L.F.; Dean, D.A. Extracellular and intracellular barriers to non-viral gene transfer. In Novel Gene Therapy Approaches; Wei, M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 75–88. ISBN 978-953-51-0966-2.
- Konishi, M.; Kawamoto, K.; Izumikawa, M.; Kuriyama, H.; Yamashita, T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008, 10, 610–618.
- Zhao, Y.; Huang, L. Lipid nanoparticles for gene delivery. In Advances in Genetics; Huang, L., Liu, D., Wagner, E., Eds.; Academic Press Inc.: San Diego, CA, USA, 2014; Volume 88, pp. 13–36. ISBN 9780128001486.
- Pahle, J.; Walther, W. Vectors and strategies for nonviral cancer gene therapy. Expert Opin. Biol. Ther. 2016, 16, 443–461.
- Junquera, E.; Aicart, E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv. Colloid Interface Sci. 2016, 233, 161–175.
- Nordling-David, M.M.; Golomb, G. Gene delivery by liposomes. Isr. J. Chem. 2013, 53, 737–747.
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450.
- Mikheev, A.A.; Shmendel, E.V.; Zhestovskaya, E.S.; Nazarov, G.V.; Maslov, M.A. Сationic liposomes as delivery systems for nucleic acids. Fine Chem. Technol. 2020, 15, 7–27.
- Mochizuki, S.; Kanegae, N.; Nishina, K.; Kamikawa, Y.; Koiwai, K.; Masunaga, H.; Sakurai, K. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim. Biophys. Acta Biomembr. 2013, 1828, 412–418.
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137.
- Zidovska, A.; Evans, H.M.; Ahmad, A.; Ewert, K.K.; Safinya, C.R. The role of cholesterol and structurally related molecules in enhancing transfection of cationic liposome-DNA complexes. J. Phys. Chem. B 2009, 113, 5208–5216.
- Schaffer, D.V.; Lauffenburger, D.A. Targeted synthetic gene delivery vectors. Curr. Opin. Mol. Ther. 2000, 2, 155–161.
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2012, 4, 219–233.
- Hattori, Y.; Nakamura, M.; Takeuchi, N.; Tamaki, K.; Shimizu, S.; Yoshiike, Y.; Taguchi, M.; Ohno, H.; Ozaki, K.I.; Onishi, H. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J. Drug Target. 2019, 27, 217–227.
- Zhao, Z.; Yao, W.; Wang, N.; Liu, C.; Zhou, H.; Chen, H.; Qiao, W. Synthesis and evaluation of mono- and multi-hydroxyl low toxicity pH-sensitive cationic lipids for drug delivery. Eur. J. Pharm. Sci. 2019, 133, 69–78.
- Maslov, M.A.; Syicheva, E.V.; Morozova, N.G.; Serebrennikova, G.A. Cationic amphiphiles of both lipid and nonlipid nature in gene therapy. Russ. Chem. Bull. 2000, 49, 385–401.
- Bofinger, R.; Zaw-Thin, M.; Mitchell, N.J.; Patrick, P.S.; Stowe, C.; Gomez-Ramirez, A.; Hailes, H.C.; Kalber, T.L.; Tabor, A.B. Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J. Pept. Sci. 2018, 24, e3131.
- Kulkarni, J.A.; Myhre, J.L.; Chen, S.; Tam, Y.Y.C.; Danescu, A.; Richman, J.M.; Cullis, P.R. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1377–1387.
- Ju, J.; Huan, M.L.; Wan, N.; Hou, Y.L.; Ma, X.X.; Jia, Y.Y.; Li, C.; Zhou, S.Y.; Zhang, B. Le Cholesterol derived cationic lipids as potential non-viral gene delivery vectors and their serum compatibility. Bioorganic Med. Chem. Lett. 2016, 26, 2401–2407.
- Cui, S.H.; Zhi, D.F.; Zhao, Y.N.; Chen, H.Y.; Meng, Y.; Zhang, C.M.; Zhang, S.B. Cationic lioposomes with folic acid as targeting ligand for gene delivery. Bioorganic Med. Chem. Lett. 2016, 26, 4025–4029.
- Hiwale, A.A.; Voshavar, C.; Dharmalingam, P.; Dhayani, A.; Mukthavaram, R.; Nadella, R.; Sunnapu, O.; Gandhi, S.; Naidu, V.G.M.; Chaudhuri, A.; et al. Scaling the effect of hydrophobic chain length on gene transfer properties of di-alkyl, di-hydroxy ethylammonium chloride based cationic amphiphiles. RSC Adv. 2017, 7, 25398–25405.
- Li, B.; Guo, W.; Zhang, F.; Liu, M.; Wang, S.; Liu, Z.; Xiang, S.; Zeng, Y. Synthesis and evaluation of L-arabinose-based cationic glycolipids as effective vectors for pDNA and siRNA in vitro. PLoS ONE 2017, 12, e0180276.
- Berchel, M.; Akhter, S.; Berthe, W.; Gonçalves, C.; Dubuisson, M.; Pichon, C.; Jaffrès, P.A.; Midoux, P. Synthesis of α-amino-lipophosphonates as cationic lipids or co-lipids for DNA transfection in dendritic cells. J. Mater. Chem. B 2017, 5, 6869–6881.
- Medvedeva, D.A.; Maslov, M.A.; Serikov, R.N.; Morozova, N.G.; Serebrenikova, G.A.; Sheglov, D.V.; Latyshev, A.V.; Vlassov, V.V.; Zenkova, M.A. Novel cholesterol-based cationic lipids for gene delivery. J. Med. Chem. 2009, 52, 6558–6568.
- Stewart, L.; Manvell, M.; Hillery, E.; Etheridge, C.J.; Cooper, R.G.; Stark, H.; Van-Heel, M.; Preuss, M.; Alton, E.W.F.W.; Miller, A.D. Physico-chemical analysis of cationic liposome-DNA complexes (lipoplexes) with respect to in vitro and in vivo gene delivery efficiency. J. Chem. Soc. Perkin Trans. 2001, 2, 624–632.
- Seiler, N.; Delcros, J.G.; Moulinoux, J.P. Polyamine transport in mammalian cells. An update. Int. J. Biochem. Cell Biol. 1996, 28, 843–861.
- Gardner, R.A.; Belting, M.; Svensson, K.; Phanstiel, O.; Iv, O.P. Synthesis and Transfection Efficiencies of New Lipophilic Polyamines. J. Med. Chem. 2007, 50, 308–318.
- Gruneich, J.A.; Diamond, S.L. Synthesis and structure-activity relationships of a series of increasingly hydrophobic cationic steroid lipofection reagents. J. Gene Med. 2007, 9, 381–391.
- Randazzo, R.A.S.; Bucki, R.; Janmey, P.A.; Diamond, S.L. A series of cationic sterol lipids with gene transfer and bactericidal activity. Bioorganic Med. Chem. 2009, 17, 3257–3265.
- Ahmed, O.A.A.; Adjimatera, N.; Pourzand, C.; Blagbrough, I.S. N4,N9-Dioleoyl Spermine Is a Novel Nonviral Lipopolyamine Vector for Plasmid DNA Formulation. Pharm. Res. 2005, 22, 972–980.
- Ghonaim, H.M.; Ahmed, O.A.A.; Pourzand, C.; Blagbrough, I.S. Varying the chain length in N4, N9-diacyl spermines: Non-viral lipopolyamine vectors for efficient plasmid DNA formulation. Mol. Pharm. 2008, 5, 1111–1121.
- Ahmed, O.A.A.; Pourzand, C.; Blagbrough, I.S. Varying the unsaturation in N4,N9-dioctadecanoyl spermines: Nonviral lipopolyamine vectors for more efficient plasmid DNA formulation. Pharm. Res. 2006, 23, 31–40.
- Soltan, M.K.; Ghonaim, H.M.; El Sadek, M.; Kull, M.A.; El-Aziz, L.A.; Blagbrough, I.S. Design and synthesis of N4,N9-disubstituted spermines for non-viral siRNA delivery—Structure-activity relationship studies of sifection efficiency versus toxicity. Pharm. Res. 2009, 26, 286–295.
- Blagbrough, I.S.; Metwally, A.A.; Ghonaim, H.M. Asymmetrical N4,N9-diacyl spermines: SAR studies of nonviral lipopolyamine vectors for efficient siRNA delivery with silencing of EGFP reporter gene. Mol. Pharm. 2012, 9, 1853–1861.
- Ghonaim, H.M.; Li, S.; Blagbrough, I.S. N1,N12-diacyl spermines: SAR studies on non-viral lipopolyamine vectors for plasmid DNA and siRNA formulation. Pharm. Res. 2010, 27, 17–29.
- Viola, J.R.; Leijonmarck, H.; Simonson, O.E.; Oprea, I.I.; Frithiof, R.; Purhonen, P.; Moreno, P.M.D.; Lundin, K.E.; Strömberg, R.; Smith, C.I.E. Fatty acid-spermine conjugates as DNA carriers for nonviral in vivo gene delivery. Gene Ther. 2009, 16, 1429–1440.
- Petukhov, I.A.; Maslov, M.A.; Morozova, N.G.; Serebrennikova, G.A. Synthesis of polycationic lipids based on cholesterol and spermine. Russ. Chem. Bull. 2010, 59, 260–268.
- Maslov, M.A.; Kabilova, T.O.; Petukhov, I.A.; Morozova, N.G.; Serebrennikova, G.A.; Vlassov, V.V.; Zenkova, M.A. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J. Control. Release 2012, 160, 182–193.
- Markov, O.O.; Mironova, N.L.; Maslov, M.A.; Petukhov, I.A.; Morozova, N.G.; Vlassov, V.V.; Zenkova, M.A. Novel cationic liposomes provide highly efficient delivery of DNA and RNA into dendritic cell progenitors and their immature offsets. J. Control. Release 2012, 160, 200–210.
- Markov, O.V.; Mironova, N.L.; Shmendel, E.V.; Serikov, R.N.; Morozova, N.G.; Maslov, M.A.; Vlassov, V.V.; Zenkova, M.A. Multicomponent mannose-containing liposomes efficiently deliver RNA in murine immature dendritic cells and provide productive anti-tumour response in murine melanoma model. J. Control. Release 2015, 213, 45–56.
- Kabilova, T.O.; Shmendel, E.V.; Gladkikh, D.V.; Chernolovskaya, E.L.; Markov, O.V.; Morozova, N.G.; Maslov, M.A.; Zenkova, M.A. Targeted delivery of nucleic acids into xenograft tumors mediated by novel folate-equipped liposomes. Eur. J. Pharm. Biopharm. 2018, 123, 59–70.
- Shmendel, E.V.; Kabilova, T.O.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Targeted delivery of nucleic acids by folate-containing liposomes into KB-3-1 and HEK 293 cells. Russ. J. Bioorganic Chem. 2019, 45, 719–725.
- Shmendel, E.; Kabilova, T.; Morozova, N.; Zenkova, M.; Maslov, M. Effects of spacers within a series of novel folate-containing lipoconjugates on the targeted delivery of nucleic acids. J. Drug Deliv. Sci. Technol. 2020, 57, 101609.
- Kabilova, T.O.; Sen’kova, A.V.; Nikolin, V.P.; Popova, N.A.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. Antitumor and antimetastatic effect of small immunostimulatory RNA against B16 melanoma in mice. PLoS ONE 2016, 11, e0150751.
- Markov, O.V.; Mironova, N.L.; Shmendel, E.V.; Maslov, M.A.; Zenkova, M.A. Systemic delivery of complexes of melanoma RNA with mannosylated liposomes activates highly efficient murine melanoma-specific cytotoxic T cells in vivo. Mol. Biol. 2017, 51, 102–107.
- Puchkov, P.A.; Kartashova, I.A.; Shmendel, E.V.; Luneva, A.S.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Spacer structure and hydrophobicity influences transfection activity of novel polycationic gemini amphiphiles. Bioorganic Med. Chem. Lett. 2017, 27, 3284–3288.
- Puchkov, P.A.; Perevoshchikova, K.A.; Kartashova, I.A.; Luneva, A.S.; Kabilova, T.O.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Polycationic amphiphiles based on triethylenetetramine and their transfection efficacy. Russ. J. Bioorganic Chem. 2017, 43, 561–569.
- Radchatawedchakoon, W.; Krajarng, A.; Niyomtham, N.; Watanapokasin, R.; Yingyongnarongkul, B. High transfection efficiency of cationic lipids with asymmetric acyl-Cholesteryl hydrophobic tails. Chem. A Eur. J. 2011, 17, 3287–3295.
- Wang, X.L.; Ramusovic, S.; Nguyen, T.; Lu, Z.R. Novel polymerizable surfactants with pH-sensitive amphiphilicity and cell membrane disruption for efficient siRNA delivery. Bioconjug. Chem. 2007, 18, 2169–2177.
- Wang, X.L.; Nguyen, T.; Gillespie, D.; Jensen, R.; Lu, Z.R. A multifunctional and reversibly polymerizable carrier for efficient siRNA delivery. Biomaterials 2008, 29, 15–22.
- Xu, R.; Lu, Z.R. Design, synthesis and evaluation of spermine-based pH-sensitive amphiphilic gene delivery systems: Multifunctional non-viral gene carriers. Sci. China Chem. 2011, 54, 359–368.
- Xu, R.Z.; Wang, X.L.; Lu, Z.R. Intracellular siRNA delivery with novel spermine based surfactants. Chin. Sci. Bull. 2012, 57, 3979–3984.
- Malamas, A.S.; Gujrati, M.; Kummitha, C.M.; Xu, R.; Lu, Z.R. Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J. Control. Release 2013, 171, 296–307.
- Dewa, T.; Asai, T.; Tsunoda, Y.; Kato, K.; Baba, D.; Uchida, M.; Sumino, A.; Niwata, K.; Umemoto, T.; Iida, K.; et al. Liposomal polyamine-dialkyl phosphate conjugates as effective gene carriers: Chemical structure, morphology, and gene transfer activity. Bioconjug. Chem. 2010, 21, 844–852.
- Asai, T.; Matsushita, S.; Kenjo, E.; Tsuzuku, T.; Yonenaga, N.; Koide, H.; Hatanaka, K.; Dewa, T.; Nango, M.; Maeda, N.; et al. Dicetyl phosphate-tetraethylenepentamine-based liposomes for systemic siRNA delivery. Bioconjug. Chem. 2011, 22, 429–435.
- Yonenaga, N.; Kenjo, E.; Asai, T.; Tsuruta, A.; Shimizu, K.; Dewa, T.; Nango, M.; Oku, N. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J. Control. Release 2012, 160, 177–181.
- Kenjo, E.; Asai, T.; Yonenaga, N.; Ando, H.; Ishii, T.; Hatanaka, K.; Shimizu, K.; Urita, Y.; Dewa, T.; Nango, M.; et al. Systemic delivery of small interfering RNA by use of targeted polycation liposomes for cancer therapy. Biol. Pharm. Bull. 2013, 36, 287–291.
- Patil, S.P.; Yi, J.W.; Bang, E.K.; Jeon, E.M.; Kim, B.H. Synthesis and efficient siRNA delivery of polyamine-conjugated cationic nucleoside lipids. Medchemcomm 2011, 2, 505–508.
- Yang, H.W.; Yi, J.W.; Bang, E.K.; Jeon, E.M.; Kim, B.H. Cationic nucleolipids as efficient siRNA carriers. Org. Biomol. Chem. 2011, 9, 291–296.
- Mével, M.; Kamaly, N.; Carmona, S.; Oliver, M.H.; Jorgensen, M.R.; Crowther, C.; Salazar, F.H.; Marion, P.L.; Fujino, M.; Natori, Y.; et al. DODAG; a versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J. Control. Release 2010, 143, 222–232.
- Mével, M.; Breuzard, G.; Yaouanc, J.J.; Clément, J.C.; Lehn, P.; Pichon, C.; Jaffrès, P.A.; Midoux, P. Synthesis and transfection activity of new cationic phosphoramidate lipids: High efficiency of an imidazolium derivative. ChemBioChem 2008, 9, 1462–1471.
- Mével, M.; Neveu, C.; Gonçalves, C.; Yaouanc, J.-J.; Pichon, C.; Jaffrès, P.-A.; Midoux, P. Novel neutral imidazole-lipophosphoramides for transfection assays. Chem. Commun. 2008, 3124–3126.
- Aissaoui, A.; Chami, M.; Hussein, M.; Miller, A.D. Efficient topical delivery of plasmid DNA to lung in vivo mediated by putative triggered, PEGylated pDNA nanoparticles. J. Control. Release 2011, 154, 275–284.
- Kim, B.; Doh, K.; Hyeung, J.; Kang, H.; Park, J.; Moon, I.; Seu, Y. Bioorganic & Medicinal Chemistry Letters Synthesis of novel cholesterol-based cationic lipids for gene delivery. Bioorg. Med. Chem. Lett. 2009, 19, 2986–2989.
- Kim, B.K.; Seu, Y.B.; Bae, Y.U.; Kwak, T.W.; Kang, H.; Moon, I.J.; Hwang, G.B.; Park, S.Y.; Doh, K.O. Efficient delivery of plasmid DNA using cholesterol-based cationic lipids containing polyamines and ether linkages. Int. J. Mol. Sci. 2014, 15, 7293–7312.
- Kim, B.K.; Doh, K.O.; Bae, Y.U.; Seu, Y.B. Synthesis and optimization of cholesterol-based diquaternary ammonium gemini surfactant (Chol-GS) as a new gene delivery vector. J. Microbiol. Biotechnol. 2011, 21, 93–99.
- Sparks, J.; Slobodkin, G.; Matar, M.; Congo, R.; Ulkoski, D.; Rea-Ramsey, A.; Pence, C.; Rice, J.; McClure, D.; Polach, K.J.; et al. Versatile cationic lipids for siRNA delivery. J. Control. Release 2012, 158, 269–276.
- Paecharoenchai, O.; Niyomtham, N.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Yingyongnarongkul, B.; Opanasopit, P. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech 2012, 13, 1302–1308.
- Niyomtham, N.; Apiratikul, N.; Suksen, K.; Opanasopit, P.; Yingyongnarongkul, B. Synthesis and in vitro transfection efficiency of spermine-based cationic lipids with different central core structures and lipophilic tails. Bioorg. Med. Chem. Lett. 2015, 25, 496–503.
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008, 26, 561–569.
- Mahon, K.P.; Love, K.T.; Whitehead, K.A.; Qin, J.; Akinc, A.; Leshchiner, E.; Leshchiner, I.; Langer, R.; Anderson, D.G. Combinatorial approach to determine functional group effects on lipidoid-mediated siRNA delivery. Bioconjug. Chem. 2010, 21, 1448–1454.
- Park, H.-J.; Jeon, E.J.; Lee, J.S.; Hong, S.H.; Cho, A.-N.; Lee, J.; Moon, J.-S.; Jung, K.-E.; Oh, J.-W.; Lee, H.; et al. Galactosylated lipidoid nanoparticles for delivery of small interfering RNA to inhibit Hepatitis C viral replication in vivo. Adv. Healthc. Mater. 2016, 1–11.
This entry is offline, you can click here to edit this entry!
