The natural polymer chitosan is the second most abundant biopolymer on earth after chitin and has been extensively explored for preparation of versatile drug delivery systems. The presence of two distinct reactive functional groups (an amino group at C2, and a primary and secondary hydroxyl group at C3 and C6) of chitosan are involved in the transformation of expedient derivatives such as acylated, alkylated, carboxylated, quaternized and esterified chitosan. Amongst these, quaternized chitosan is preferred in pharmaceutical industries owing to its prominent features including superior water solubility, augmented antimicrobial actions, modified wound healing, pH-sensitive targeting, biocompatibility, and biodegradability. It has been explored in a large realm of pharmaceuticals, cosmeceuticals, and the biomedical arena. Immense classy drug delivery systems containing quaternized chitosan have been intended for tissue engineering, wound healing, gene, and vaccine delivery.
- quaternized chitosan
- antimicrobial
- biocompatibility
- biomedical
- vaccine
- wound healing
1. Introduction
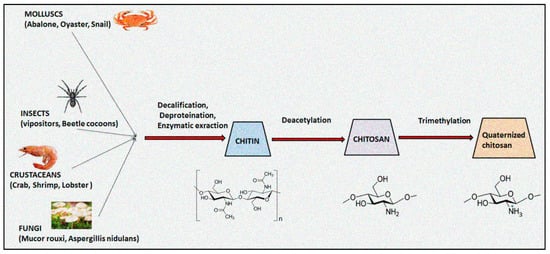
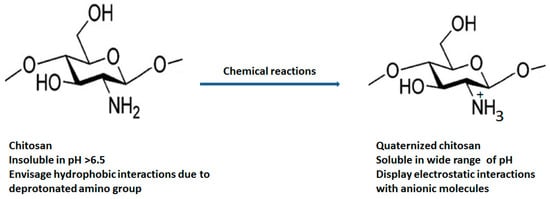
2. Quaternized Chitosan Derivatives and Physicochemical Properties
|
Parameters |
Responses |
|---|---|
|
Degree of quaternization (<65%) |
Increased cytotoxicity |
|
Increased mucoadhesiveness |
|
|
Decreased anticoagulation effect |
|
|
Degree of quaternization (≥20%) |
Increased antimicrobial action in pH = 7.2 |
|
No effect on antimicrobial action in acidic pH |
|
|
Degree of substitution (<1%) |
Increased antioxidant property |
|
Degree of substitution (<25%) |
Increased antithrombin action and acid-binding capacity |
|
Degree of substitution (>1%) |
Decreased moisture absorption and retention ability |
|
High concentration |
Increased particle size, aggregation, zeta potential, cytotoxicity |
|
Decreased knockdown efficiency and poor transfection efficacy |
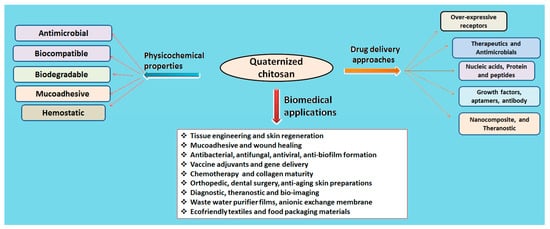
3. Biomedical Applications of Quaternized Chitosan Derivatives
3.1. Antimicrobial
3.2. Antiproliferative
3.3. Antibiofilm
3.4. Antifungal Activity
3.5. Mucoadhesiveness
3.6. Drug Carriers
3.6.1. Nanofibers
|
Components |
Purpose |
Research Outcomes |
References |
|---|---|---|---|
|
HTCC and PVA |
Retention of non-enveloped virus on the highly charged HTCC/PVA nanofibers. |
Nano-scaled HTCC/PVA nanofibers (100–200 nm) were developed, having the potential to adsorb mammalian virus porcine parvovirus (95%). The developed system followed Freundlich isotherm and showed fast adsorption kinetics (pseudo first order), which suggested the formation of efficient filter material for the purification of water |
[57] |
|
Doxorubicin, poly (L-lactide-coD, L-lactide) and QCh |
Doxorubicin embedded poly (L-lactide-coD, L-lactide) mats were modified with QCh to enhance anti-proliferative activity. |
Developed mats were evaluated against human breast carcinoma cell lines (MCF-7) and exhibited reduced cell viability and amplified antiproliferative activity. Fluorescent microscopy revealed that the presence of QCh induced apoptosis, which was the primary mechanism of MCF-7 cell death. |
[58] |
|
2,3-Epoxy-propyl trimethyl ammonium chloride |
QCh fibres were designed using 2,3-epoxypropyl trimethyl. Ammonium chloride following ring open reaction to modify antibacterial and liquid absorption capacity. |
Outcomes revealed excellent water retention capacity, modified swelling index and mechanical strength compared to bare chitosan. Superior antibacterial efficacy against S. aureus and lower cytotoxicity suggested its vital role in fabricating wound dressing materials. |
[59] |
|
Poly (lactic acid), QCh |
Stereo complex crystallite (SC) membrane containing poly (lactic acid) QCh were employed to design disinfectant wound dressing material. |
The enhanced thermal and mechanical properties of developed SC membrane owing to restricted mobility of lactide chains. They have better wound healing capacity (100% in 15 days). This biomass-based membrane was multifunctional as it has antioxidant, antibacterial and wound healing efficacy. |
[60] |
|
Silica coated poly (vinylidene) fluoride and QCh |
High-performance anion exchange silica-coated (vinylidene) fluoride along with QCh nanofibrous membrane were designed. |
The surface of silica-coated poly (vinylidene) fluoride was grafted with quaternized chitosan to pursue dual action, i.e., ion exchange and strong reinforcement substrate. QCh-impregnated nanofibers showed superb mechanical strength (11.9Mpa). Adorned positive charges created channel-like ion transport channels that could efficiently serve as anion exchange membrane. |
[61] |
3.6.2. Hydrogel
|
Objective |
Components |
Research Highlights |
References |
|---|---|---|---|
|
Multifunctional QCh-based polyacrylamide hydrogel was developed that contained hemostatic and skin adhesive properties |
QCh, Matrigel-polyacrylamide |
The developed hybrid hydrogel had a three-dimensional microporous integrity and exhibited high mechanical strength and good adhesiveness with low toxicity. The outcomes from the histology study demonstrated improvement in wound healing, collagen deposition, and stimulation of skin adnexal regeneration. The developed QCh-based antibacterial hydrogel demonstrated promising potential for designing wound dressing materials. |
[63] |
|
Dual crosslinked QCh-clindamycin loaded hydrogel was prepared to manage methicillin-resistant S. aureus (MRSA) bacteria |
QCh, clindamycin |
The developed nanocomposite-embedded hydrogel withstood sufficient mechanical and injectable efficiencies. The system responded on variable pH that enabled maximum interaction with MRSA bacteria (90% killed) in acidic conditions and overcame the antibiotic resistance challenge. |
[64] |
|
A novel wound dressing-based injectable hydrogel was designed employing QCh and PLEL (PLEL-nBG-QCS-C) hydrogel to promote angiogenesis. |
QCh and PLEL [Poly (D, L-lactide)-poly (ethylene glycol)-poly (D,L-lactide)] and bioactive glass |
PLEL hydrogels preloaded with bioactive glass (CaO-SiO2-P2O5) could efficiently seal the broken skin and increase the cure rate of wounds. Additionally, they were thermosensitive, tissue adhesive, and antibacterial. |
[65] |
|
QCh-based timolol maleate thermosensitive hydrogel was prepared for improved ophthalmic disorders. |
Timolol maleate, Sodium hydrogen carbonate, QCh |
The developed transparent thermosensitive hydrogel presented desirable porosity, swelling index, and biodegradability. The addition of sodium hydrogen carbonate enabled enhanced thermosensitivity to the system. In vitro drug release revealed the initial burst release in early hours followed by controlled release of timolol maleate for a week. This supported the potential use of the developed hydrogel for glaucoma management. |
[66] |
|
Dopamine-gelatin-crosslinked QCh injectable hydrogel was prepared to localize delivery for the combat of Parkinson and associated inflammation as well. |
Dopamine, QCh, Metronidazole, gelatin |
The formulated injectable hydrogel exhibited sufficient rheological parameters. The cytocompatibility of hydrogel revealed the cell viability and proliferation of L929 fibroblast cells. In vitro study exposed localized release of both dopamine and metronidazole. |
[67] |
|
QCh-based pH-sensitive veterinary hydrogel vaccine for improved cellular and humoral responses. |
QCh, MontanideTM ISA206 and glycerophosphate |
The developed hydrogel was biocompatible, safe, and had efficiencies to adsorb inactivated porcine reproductive and respiratory syndrome virus. Moreover, the system ruled out the downsides of mineral oil side effects and encouraged immunogenicity. |
[68] |
|
Development of NQC-loaded thermostable and multifunctional hydrogel |
N-quaternized chitosan (NQC), poly vinyl alcohol, glutaraldehyde |
Different hydrogels on varying concentration of NQC and PVA were designed to modify metal ion uptake, swelling capacity, compatibility, and antibacterial efficacy. |
[69] |
3.6.3. Beads
3.6.4. Nanoparticles
|
Objective |
Components |
Research Highlights |
References |
|---|---|---|---|
|
Ketoconazole was entrapped in QCh NPs for superior antifungal activity |
Ketoconazole, QCh, sodium triphosphate |
Nanoscaled KCZ-QCSNPs displayed superb entrapment efficiency (~90%). Performed tube dilution method revealed preeminent antimicrobial activity. |
[75] |
|
QCh derivative ‘HTCC’ NPs were embedded in various fabric materials to evaluate antimicrobial efficacy. |
HTCC, cotton fabric, polyester, polyacrylic acid |
The developed HTCC nanoparticles embedded in cotton fabric exhibited superior antimicrobial action against Fusarium oxysporum and Bacillus subtilis compared to polyester and mixture of cotton. |
[76] |
|
Anthrax vaccine adjuvant containing Fucoidan-HTCC nanoparticles were developed to improve rapid induction of immunity |
Sulphated polysaccharide (Fucoidan, FUC) and HTCC |
An active complexation between opposite-charged FUC and HTCC was conducted through varying their mass ratio. MTT assay on L929 or JAWS dendritic cells evaluated low cytotoxicity, improved cellular internalization and high cell viability. Combination of FUC-HTCCNPs and anthrax vaccine adsorbed (AVA) significantly improved magnitude of cellular/humoral immunity and mice survival rate compared to administration of AVA alone. |
[77] |
|
Nanoparticles containing N-2-HTCC and N,O-CMC encapsulated vaccine antigens (IBV/H120) were developed for significant increments in lymphocytes, interleukins, and interferon in chicken |
N-2-HTCC, N,O-carboxy methyl chitosan (CMC), infectious bronchitis virus (IBV)/H120 and Newcastle disease virus (NDV) |
The developed nanoparticles, i.e., N-2-HTCC-CMC/NDV/IBV, predicted great stability and low cytotoxicity on storing at 37 °C for 3 weeks. In vivo assay on chicken revealed sustained release of both NDV and IBV with enhanced release of IgG and IgA that facilitated the proliferation of immune modifiers in chicken body. The developed QCh-based NPs showed the potential to combat respiratory diseases in chicken. |
[78] |
|
Ecofriendly QCh derivative HTCC nanoparticles were designed to increase the durability and microbial resistance of Antheraea pernyi silk fabric. |
HTCC and 1,2,3,4 butane tetracarboxylic acid, sodium hypophosphite |
The conventional dip-and-dry-cure method was applied to evaluate silk fabric durability (A. pernyi). Wrinkle resistance, microbial resistance (against S. aureus and E. coli) and shrinkage resistance were observed even after washing A. pernyi silk fabric more than 50 times. |
[79] |
|
5-flurouracil (5-FU) embedded HTCC NPs developed for improved entrapment efficiency and in vitro release |
5-FU, HTCC, sodium tripoly-phosphate (TPP) |
5-FU/HTCC NPs were prepared through ionic gelation method via electrostatic interaction between positive-charged HTCC and negative-charged TPP. Encapsulated 5-FU exhibited controlled release profile in pH 7.4 buffer. |
[80] |
3.6.5. Quaternized Chitosan Nanocomposites
3.6.6. Vaccine Adjuvants
This entry is adapted from the peer-reviewed paper 10.3390/polym13152514
References
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051.
- Kumar, A.; Kumar, A. The virtuous potential of chitosan oligosaccharide for promising biomedical applications. J. Mater. Res. 2020, 35, 1123–1134.
- Islam, S.M.A.; Bhuvian, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866.
- Kahya, N. Water Soluble Chitosan Derivatives and their Biological Activities: A Review. Polym. Sci. 2019, 5, 1–16.
- Kofuji, K.; Qian, C.; Nishimura, M.; Sugiyama, I.; Murata, Y.; Kawashima, S. Relationship between physicochemical characteristics and functional properties of chitosan. Eur. Polym. J. 2005, 41, 2784–2791.
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872.
- Yuan, Y.; Betsy, M.; Chesnutt, W.; Haggard, O.; Bumgardner Joel, D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416.
- Marques, C.; Som, C.; Schmutz, M.; Borges, O.; Borchard, G. How the Lack of Chitosan Characterization Precludes Implementation of the Safe-by-Design Concept. Front. Bioeng. Biotech. 2020, 8, 165.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120 Pt A, 1181–1189.
- Kumari, S.; Kumar, A.S.H.; Abanti, S.; Kumar, R.P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104 Pt B, 1697–1705.
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678.
- Cai, J.; Dang, Q.; Liu, C.; Fan, B.; Yan, J.; Xu, Y.; Li, J. Preparation and characterization of N-benzoyl-O-acetyl-chitosan. Int. J. Biol. Macromol. 2015, 77, 52–58.
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126.
- Mohammadi, E.; Daraei, H.; Ghanbari, R.; Dehestani Athar, S.; Zandsalimi, Y.; Ziaee, A.; Maleki, A.; Yetilmezsoy, K. Synthesis of carboxylated chitosan modified with ferromagnetic nanoparticles for adsorptive removal of fluoride, nitrate, and phosphate anions from aqueous solutions. J. Mol. Liq. 2019, 273, 116–124.
- Benediktsdóttir, B.E.; Baldursson, Ó.; Másson, M. Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application. J. Control. Release 2014, 173, 18–31.
- Ramasamy, P.; Subhapradha, N.; Thinesh, T.; Selvin, J.; Selvan, K.M.; Shanmugam, V.; Shanmugam, A. Characterization of bioactive chitosan and sulfated chitosan from Doryteuthis singhalensis (Ortmann, 1891). Int. J. Biol. Macromol. 2017, 99, 682–691.
- Cao, J.; You, J.; Zhang, L.; Zhou, J. Homogeneous synthesis and characterization of chitosan ethers prepared in aqueous alkali/urea solutions. Carbohydr. Polym. 2018, 185, 138–144.
- Jia, Z.; Shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6.
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 1, 141–149.
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901.
- Aranaz, I.; Mengíbar, M.; Harris, R.; Miralles, B.; Acosta, N.; Calderon, L.; Sanchez, A.; Heras, A. Role of Physicochemical Properties of Chitin and Chitosan on their Functionality. Curr. Chem. Biol. 2014, 8, 27–42.
- Chen, K.; Guo, B.; Luo, J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr. Polym. 2017, 173, 100–106.
- Jana, S.; Florczyk, S.; Leung, M.; Zhang, M. High-strength pristine porous chitosan scaffolds for tissue engineering. J. Mater. Chem. 2012, 22, 6291–6299.
- Diabb Zavala, J.M.; Leija Gutiérrez, H.M.; Segura-Cárdenas, E.; Mamidi, N.; Morales-Avalos, R. Manufacture and mechanical properties of knee implants using SWCNTs/UHMWPE composites. J. Mech. Behav. Biomed. Mater. 2021, 120, 104554.
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291.
- Britto, D.; Assis, O. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 2007, 41, 198–203.
- Mamidi, N.; Velasco Delgadillo, R.M.; Gonzáles Ortiz, A.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208.
- Mamidi, N.; Velasco Delgadillo, R.M.; Castrejon, J.V. Unconventional and facile production of a stimuli-responsive multifunctional system for simultaneous drug delivery and environmental remediation. Environ. Sci. Nano 2021, 8, 2081–2097.
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450.
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Mar. Drugs 2014, 12, 4635–4658.
- Jarmila, V.; Vavríková, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities-a review. Curr. Pharm. Des. 2011, 17, 3596–3607.
- Sajomsang, W.; Gonil, P.; Saesoo, S. Synthesis and antibacterial activity of methylated N-(4-N,N-dimethylaminocinnamyl) chitosan chloride. Eur. Polym. J. 2009, 45, 2319–2328.
- Avadi, M.R.; Sadeghi, A.M.M.; Tahzibi, A.; Bayati, K.; Pouladzadeh, M.; Zohuriaan-Mehr, M.J.; Rafiee-Tehrani, M. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. Eur. Polym. J. 2004, 40, 1355–1361.
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306.
- Vallapa, N.; Wiarachai, O.; Thongchul, N.; Pan, J.; Tangpasuthadol, V.; Kiatkamjornwong, S.; Hoven, V.P. Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization. Carbohydr. Polym. 2011, 83, 868–875.
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936.
- Goodman, M.; Naiman, D.Q.; Lakind, J.S. Systematic review of the literature on triclosan and health outcomes in humans. Crit. Rev. Toxicol. 2018, 48, 1–51.
- Nakamura, T.; Kashimura, N.; Noji, T.; Suzuki, O.; Ambo, Y.; Nakamura, F.; Kishida, A. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: A randomized controlled trial. Surgery 2013, 153, 576–583.
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298.
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized Chitosan as an Antimicrobial Agent: Antimicrobial Activity, Mechanism of Action and Biomedical Applications in Orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869.
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409.
- Patel, R. Biofilm and antimicrobial resistance. Clin. Orthop. Relat. Res. 2005, 437, 41–47.
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.; Pereira, A.G.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832.
- Zaha, D.C.; Bungau, S.; Uivarosan, D.; Tit, D.M.; Maghiar, T.A.; Maghiar, O.; Pantis, C.; Fratila, O.; Rus, M.; Vesa, C.M. Antibiotic Consumption and Microbiological Epidemiology in Surgery Departments: Results from a Single Study Center. Antibiotics 2020, 9, 81.
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Uccello-Barretta, G.; Chetoni, P. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-_-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42.
- Li, M.Q.; Chen, X.G.; Liu, J.M.; Zhang, W.F.; Tang, X.X. Molecular weight-dependent antifungal activity and action mode of chitosan against Fulvia fulva (Cooke) Ciffrri. J. Appl. Polym. Sci. 2011, 119, 3127–3135.
- Guo, Z.Y.; Xing, R.G.; Liu, S.; Zhong, Z.M.; Ji, X.; Wang, L.; Li, P.C. The influence of the cationic of quaternized chitosan on antifungal activity. Int. J. Food Microbiol. 2007, 118, 214–217.
- Guo, Z.Y.; Xing, R.E.; Liu, S.; Zhong, Z.M.; Ji, X.; Wang, L.; Li, P.C. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr. Polym. 2008, 71, 694–697.
- Chethan, P.D.; Vishalakshi, B.; Sathish, L.; Ananda, K.; Poojary, B. Preparation of substituted quaternized arylfuran chitosan derivatives and their antimicrobial activity. Int. J. Biol. Macromol. 2013, 59, 158–164.
- de Oliveira Pedro, R.; Takaki, M.; Gorayeb, T.C.; Del Bianchi, V.L.; Thomeo, J.C.; Tiera, M.J.; de Oliveira Tiera, V.A. Synthesis, characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus. Microbiol. Res. 2013, 168, 50–55.
- Il’Ina, A.V.; Shagdarova, B.T.; Lun’Kov, A.P.; Kulikov, S.N.; Varlamov, V.P. In vitro antifungal activity of metal complexes of a quaternized chitosan derivative with copper ions. Microbiology 2017, 86, 590–595.
- De Souza, R.H.F.V.; Takaki, M.; Pedro, R.D.O.; Gabriel, J.D.S.; Tiera, M.J.; Tiera, V.A.D.O.; De Souza, R.V. Hydrophobic effect of amphiphilic derivatives of chitosan on the antifungal activity against Aspergillus flavus and Aspergillus parasiticus. Molecules 2013, 18, 4437–4450.
- Snyman, D.; Hamman, J.H.; Kotze, A.F. Evaluation of the Mucoadhesive Properties of N-Trimethyl Chitosan Chloride. Drug Dev. Ind. Pharm. 2003, 29, 61–69.
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative Amino Acids Separation through Cellulose Derivative Membranes with Microporous Polypropylene Fiber Matrix. Membranes 2021, 11, 429.
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surf. B Biointerfaces 2017, 149, 301–311.
- Bai, B.; Mi, X.; Xiang, X.; Heiden, P.A.; Heldt, C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr Res. 2013, 18, 137–142.
- Mi, X.; Heldt, C.L. Adsorption of a non-enveloped mammalian virus to functionalized nanofibers. Colloids Surf. B Biointerfaces 2014, 121, 319–324.
- Ignatova, M.; Yossifova, L.; Gardeva, E.; Manolova, N.; Toshkova, R.; Rashkov, I.; Alexandrov, M. Antiproliferative activity of nanofibers containing quaternized chitosan and/or doxorubicin against MCF-7 human breast carcinoma cell line by apoptosis. J. Bioact. Compat. Polym. 2011, 26, 539–551.
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Potential of quaternization-functionalized chitosan fiber for wound dressing. Int. J. Biol. Macromol. 2013, 52, 327–332.
- Ren, Y.; Huang, L.; Wang, Y.; Mei, L.; Fan, R.; He, M.; Wang, C.; Tong, A.; Chen, H.; Guo, G. Stereocomplexed electrospun nanofibers containing poly (lactic acid) modified quaternized chitosan for wound healing. Carbohydr. Polym. 2020, 247, 116754.
- Liu, G.; Tsen, W.C.; Jang, S.C.; Hu, F.; Zhong, F.; Zhang, B.; Wang, J.; Liu, H.; Wang, G.; Wen, S.; et al. Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells. J. Membr. Sci. 2020, 611, 118242.
- Xiao, X.; Zhu, Y.; Liao, J.; Wang, T.; Sun, W.; Tong, Z. High-efficient and synergetic antibacterial nanocomposite hydrogel with quaternized chitosan/Ag nanoparticles prepared by one-pot UV photochemical synthesis. Biopolymers 2020, 111, e23354.
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302.
- Wei, S.; Liu, X.; Zhou, J.; Zhang, J.; Dong, A.; Huang, P.; Wang, W.; Deng, L. Dual-crosslinked nanocomposite hydrogels based on quaternized chitosan and clindamycin-loaded hyperbranched nanoparticles for potential antibacterial applications. Int. J. Biol. Macromol. 2020, 155, 153–162.
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826.
- Fathi, P.; Omidi, M.-Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128.
- Ren, Y.; Zhao, X.; Liang, X.P.; Ma, X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087.
- Wang, Y.-Q.; Liu, Y.; Wang, Y.-X.; Wu, Y.-J.; Jia, P.-Y.; Shan, J.-J.; Wu, J.; Ma, G.-H.; Su, Z.-G. The potential adjuvanticity of quaternized chitosan hydrogel based microparticles for porcine reproductive and respiratory syndrome virus inactivated vaccine. Int. Immunopharm. 2016, 39, 84–91.
- Riham, R.; Mohamed Mahmoud, H.; Abu Elella, M.; Sabaa, W. Synthesis, characterization and applications of N-quaternized chitosan/poly(vinyl alcohol) hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161.
- Tan, Y.; Wu, H.; Xie, T.; Chen, L.; Hu, S.; Tian, H.; Wang, Y.; Wang, J. Characterization and antibacterial effect of quaternized chitosan anchored cellulose beads. Int. J. Biol. Macromol. 2020, 155, 1325–1332.
- Sowmya, A.; Meenakshi, S. An efficient and regenerable quaternary amine modified chitosan beads for the removal of nitrate and phosphate anions. J. Environ. Chem. Eng. 2013, 1, 906–915.
- Eskandarloo, H.; Godec, M.; Arshadi, M.; Padilla-Zakour, O.I.; Abbaspourrad, A. Multi-porous quaternized chitosan/polystyrene microbeads for scalable, efficient heparin recovery. Chem. Eng. J. 2018, 348, 399–408.
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B Biointerfaces 2012, 91, 168–174.
- Li, Q.; Wang, W.; Hu, G.; Cui, X.; Sun, D.; Jin, Z.; Zhao, K. Evaluation of Chitosan Derivatives Modified Mesoporous Silica Nanoparticles as Delivery Carrier. Molecules 2021, 26, 2490.
- Dhiman, P.; Bhatia, M. Ketoconazole loaded quaternized chitosan nanoparticles-PVA film: Preparation and evaluation. Polym. Bull. 2021.
- Ji, Q.X.; Zhao, Q.S.; Deng, J.; Lü, R. A novel injectable chlorhexidine thermosensitive hydrogel for periodontal application: Preparation, antibacterial activity and toxicity evaluation. J. Mater. Sci. Mater. Med. 2010, 21, 2435–2442.
- Chuang, C.C.; Tsai, M.H.; Yen, H.J.; Shyu, H.F.; Cheng, K.M.; Chen, X.A.; Chen, C.C.; Young, J.J.; Kau, J.H. A fucoidan-quaternary chitosan nanoparticle adjuvant for anthrax vaccine as an alternative to CpG oligodeoxynucleotides. Carbohydr. Polym. 2020, 229, 115403.
- Zhao, K.; Li, S.; Li, W.; Yu, L.; Duan, X.; Han, J.; Wang, X.; Jin, Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against Newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017, 24, 1574–1586.
- Lu, Y.; Cheng, D.; Lu, S.; Huang, F.; Li, G. Preparation of quaternary ammonium salt of chitosan nanoparticles and their textile properties on Antheraea pernyi silk modification. Textile Res. J. 2014, 84, 2115–2124.
- Wen, Y.; Zhang, X.Y.; Sheng, L.; Lian, X.J. Preparation and In Vitro Release Study of Quaternized Chitosan Nanoparticles. Adv. Mater. Res. 2014, 1053, 466–472.
- Abdel-Aziz, M.M.; Elella, M.H.A.; Mohamed, R.R. Green synthesis of quaternized chitosan/silver nanocomposites for targeting mycobacterium tuberculosis and lung carcinoma cells (A-549). Int. J. Biol. Macromol. 2020, 142, 244–253.
- Luo, J.; Han, G.; Xie, M.; Cai, Z.; Wang, X. Quaternized chitosan/montmorillonite nanocomposite resin and its adsorption behavior. Iran. Polym. J. 2015, 24, 531–539.
- Jang, S.-C.; Chuang, F.-S.; Tsen, W.-C.; Kuo, T.-W. Quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. J. Appl. Polym. Sci. 2019, 136, 47778.
- Chunli, G.; Shujun, Z.; Wen-Chin, T.; Fuqiang, H.; Fei, Z.; Bingqing, Z.; Hai, L. Hierarchical layered double hydroxide coated carbon nanotube modified quaternized chitosan/polyvinyl alcohol for alkaline direct methanol fuel cells. J Power Sources 2019, 441, 227176.
- Abueva, S.D.; Kim, C.; Lee, B.; Nath, B.T. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169.
- Li, X.; Xing, R.; Xu, C.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr. Polym. 2021, 264, 118050.
