2. Discussion
This study was performed to enable a facile system for monitoring genome editing in living animals as well as to identify edited tissue at a cellular resolution. This work shows that established cre-loxP reporter systems can be used to monitor CRISPR activity. This paper showed that loxP cleavage by SaCas9 can be comparable to cre recombinase activity in deleting stop signals in vitro or in vivo. By using the previously established mT/mG:LSL Luc, it was demonstrated that CRISPR activity could be detected non-invasively in living mice along with a quantitative approach upon dissection of edited tissues.
This system also establishes the first
loxP specific reporter for fluorescence monitoring. Previous work has used gRNAs to target adjacent to the
loxP site or within the polyA region (
Supplementary Figure S2A). While these have shown the ability to monitor CRISPR activity via tissue sectioning and DNA analysis, these systems are specific to Ai9 mice and a few related strains and would not be broadly applicable to other reporter systems such as the LSL-mice or the mT/mG target loci [
25]. Other work has shown in vitro targeting with partial overlap with
loxP and complete coverage with the gRNA in the
loxP mutants
lox71 and
lox66 (
Supplementary Figure S2B) [
26]. These previous gRNAs would not be applicable to most mouse models that likely will not have convenient adjacent sequences that allow for targeting and most mouse reporter models do not use
loxP mutants. The system shown herein can be used with any wildtype LoxP site and is therefore capable of being used with any mouse model that uses
loxP to activate or deactivate genes by cre excision. This system could also be used in cases where
loxP is positioned for gene inversion to delete a region or disrupt this process.
Cre recombinase has evolved to not only cut
loxP sequences, but to also repair the resulting genomic lesion. In contrast, Cas editors simply cleave DNA. Any Cas cleavage event must rely on the host cell to repair the cut DNA. Therefore, one might assume that Cas-mediated deletion of a floxed locus would likely be less efficient than the evolved cut and repair cre recombinase system. However, when using SaCas9 and the dual guide system, SaCas9 was like cre in efficiency for reporter activation in vitro and in vivo (
Figure 1,
Figure 4 and
Figure 5). This activity level can be partially explained by SaCas9, an efficient DNA editor that can show higher activity than SpCas9 and a Cas12a variant when using similar or identical targets [
21].
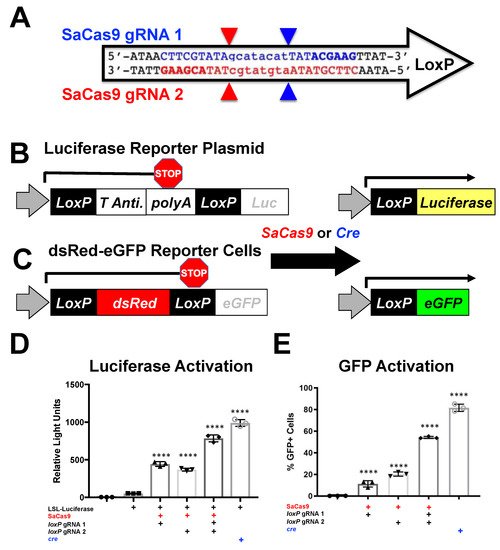
Figure 1. Gene activation through targeting loxP in vitro. (A) The loxP sequence is shown with key features and target sites for CRISPR SaCas9. The capitalized base pairs are the 13-base-pair palindromic regions flanking the 8-base-pair core that gives loxP its directionality. The gRNA 1 homologous strand to the guide RNA is depicted in light blue with the PAM-binding region in dark blue and bold letters and a triangle to indicate the cleavage site. gRNA 2 depicts the same in red. (B) This depicts the reporter plasmid p133, the LSL-Luc reporter plasmid used in D, and the general outcome when treated with SaCas9 with targeting loxP gRNAs or cre recombinase. (C) The lentiviral vector is used to make the Red/Green reporter cells used in (E) and similarly depicts the outcome when gene edited by SaCas9 or Cre. (D) Cells were plated into a 6-well plate and transfected at 60–80% confluency with Xfect and 2.5 μg of the reporter plasmid was transfected to be targeted by cre or SaCas9. Groups were compared by one-way Anova and Tukey’s multiple comparison. Using this, all the groups were significant compared to other groups except between the untransfected control group and the P133 transfected group and between the individual gRNA treated groups; 95% confidence intervals are shown and the significance against the control is shown (n = 3). (E) The Red/Green HEK293 reporter cells were transfected with 5 μg of plasmid. The single gRNA groups were significant compared to untransfected and the combination of gRNAs and cre was significant compared to all groups by one-way ANOVA with Tukey’s multiple comparisons. Individual gRNAs were not significant compared to each other (n = 3) (**** p < 0.00001).
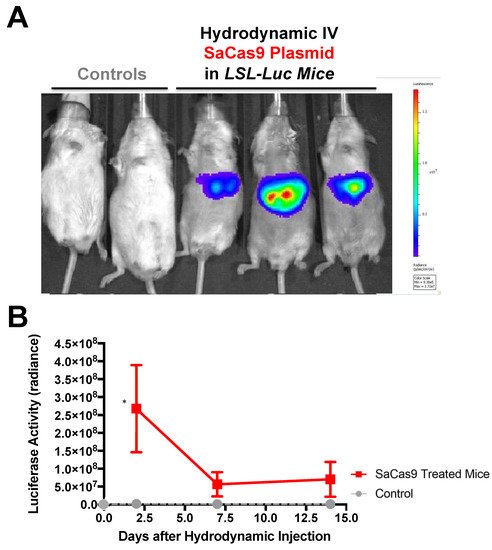
Hydrodynamic delivery functions by rupturing the cell membranes under pressure, pushing the plasmid DNA into the cell. This pressure and resulting damage could be responsible for the decline in luciferase activity from Day 2 to Day 7 as seen in Figure 3, Figure 4 and Figure 6. This coupled with the introduction of neo-antigens in the form of SaCas9 or cre could lead to immunological responses to transduced cells as well. Additionally, there is a discrepancy between the SaCas9 hydrodynamically injected mice in Figure 4; Figure 5. Older mice are not as effective for hydrodynamic delivery and older mice were used in Figure 4. Different maxi-preps were used between the experiments so this may have also contributed.
Additionally,
Figure 7 shows that there is a dose response to differing amounts of SaCas9. This is of value because it shows that this system could be used to monitor variant levels of delivery via luciferase activity. Whether SaCas9 is delivered by nanoparticle, virus, ribonucleoprotein, or some novel method, this provides a method to detect variations in delivery efficiency non-invasively and with cellular specificity. It should also be noted that the luciferase levels showed a significant decrease compared to previous experiments. We believe this to be the result of imaging differences between the Xenogen and the Lumina.
Supplementary Figure S3 also shows that this reporter system works in tissues beyond the liver. The Barry lab has also previously published in Hillestad et al. that they were able to detect cre recombinase activity using this reporter mouse in the liver, heart, lungs, muscles, brain, kidney, and spleen [
10].
Next generation sequencing analyses of the gene editing outcomes provide some insight into how the cell repairs these DNA breaks prior to reporter activation. Three repair pathways were non-homology-induced repairs, small local homology (microhomology), and homology-based outcomes, especially considering that deletions occur between two identical
loxP sequences. Analyses of deletions induced by individual gRNAs demonstrated that most of the edits result in the recreation of a
loxP site. Unfortunately, these can be the result of either NHEJ or homology-based repair when using individual gRNAs (
Supplementary Figure S1).
Analysis of the dual gRNA-induced deletions demonstrated a different mix of outcomes (
Supplementary Figure S1). After the repair pathway-ambiguous
loxP recreation, the next two top reads consisted of NHEJ-based events that are caused by different gRNAs targeting each
loxP site. Although the top reads are ambiguous, the top reads that are capable of being definitively linked to a pathway are NHEJ. Depending on which gRNA edits which
loxP site will determine whether an 8bp region would be duplicated or deleted. That being said, these cuts have the potential to cause microhomology-based events, only one of which is distinguishable as such. The strong prevalence of NHEJ-specific events strongly suggests that this is a major repair mechanism, but with the top detected outcome being ambiguous and repeated cutting of regenerated
loxP sites confusing the data further, it cannot be determined at this time what is the primary repair pathway. Further parsing of the mechanism may be done in future work by specifically knocking down proteins related to these pathways.
The sequencing was initially done to explore the possibility of alternative DNA repair pathways being responsible for the increased efficacy of both gRNAs over individual gRNAs. While repair mechanisms cannot be determined conclusively, the increases in efficacy seen with both gRNAs are potentially connected with CRISPR gene editing selecting for mutations that prevent further cutting. With a single gRNA, there is the potential for the gRNA target site to be cut and result in an insertion or deletion (indel) rather than result in a large deletion and preclude the possibility for a subsequent DSB generating a large deletion. An indel near the cut site of the gRNA target site would greatly reduce, if not inhibit, SaCas9 cutting as seen by some of the top reads in NGS [
27]. If a
loxP site is mutated to prevent CRISPR cutting at both
loxP sites, large deletion of the stop cassette would be prevented. With two potential gRNA targets, the potential indel would be further away from the PAM and less likely to inhibit SaCas9 binding [
28]. This may potentially explain the increased efficacy seen with two gRNAs rather than one.
By targeting Cas9 to the loxP site, there is also the opportunity to enable further manipulation of these sites. These models can be used in conjunction with targeted insertion technology to deliver genes of interest at loxP sites. This could be done to modify the sites to express a different gene or to reconstitute stop cassettes with mutant loxP sites that are resistant to CRISPR cutting but available to cre recombinase. There is also the potential of creating a three-outcome cassette: starting cassette, cre recombinase-treated expression cassette, and CRISPR-treated cassette. This would give greater control over animal models that would be able to turn on defective genes via cre recombinase and then deactivate them by CRISPR. There is also the potential of using the deactivated CRISPR enzyme as an inhibitor of cre recombinase, binding loxP and preventing binding and recombination. This could act to prevent cre recombinase activity in specific cell populations.
It should be noted that despite the great activity shown by SaCas9 targeting loxP, results may vary depending on the DNA site being targeted or the CRISPR being used. This SaCas9 reporter system is highly efficient while the ErCas12a has lower activity. (Figure 1 and Figure 2). This can be attributed, at least partially, by differential binding and cleavage kinetics demonstrated by Cas12a type effectors compared to Cas9. It would be of interest in the future to determine if similar Cas12a effectors such as AsCas12a or LbCas12a can improve upon this foundational work with SaCas9. It is important to note that this may overestimate the activity when using a gRNA relevant to a gene therapy application or a CRISPR other than SaCas9.
This three-way reporter system can be applied to in vivo delivery of CRISPR systems to assess the tropism of the delivery system on a broad and narrow level along with a timeline of CRISPR editing. More broadly speaking, this system can be used in combination with any loxP system that relies on deletion for its activity, for which there are over 3000 mice on JAX Laboratories website related to the cre-lox system.