Creatine is a natural nitrogenous organic acid that is integral to energy metabolism, and is crucial for proper cell functioning [8,9]. Creatine can be charged to the high-energy product phosphocreatine by creatine kinase and ATP. In the human body, the majority of creatine (>90%) is present in skeletal muscle, cardiac muscle, smooth muscle, the brain, and in the nervous tissue. Furthermore, smaller amounts are present in other tissues and cell types, including the kidney, erythrocytes, and leucocytes.
1. Overview
There is great need for the identification of new, potentially modifiable risk factors for the poor health-related quality of life (HRQoL) and of the excess risk of mortality in dialysis-dependent chronic kidney disease patients. Creatine is an essential contributor to cellular energy homeostasis, yet, on a daily basis, 1.6–1.7% of the total creatine pool is non-enzymatically degraded to creatinine and subsequently lost via urinary excretion, thereby necessitating a continuous supply of new creatine in order to remain in steady-state. Because of an insufficient ability to synthesize creatine, unopposed losses to the dialysis fluid, and insufficient intake due to dietary recommendations that are increasingly steered towards more plant-based diets, hemodialysis patients are prone to creatine deficiency, and may benefit from creatine supplementation. To avoid problems with compliance and fluid balance, and, furthermore, to prevent intradialytic losses of creatine to the dialysate, we aim to investigate the potential of intradialytic creatine supplementation in improving outcomes. Given the known physiological effects of creatine, intradialytic creatine supplementation may help to maintain creatine homeostasis among dialysis-dependent chronic kidney disease patients, and consequently improve muscle status, nutritional status, neurocognitive status, HRQoL. Additionally, we describe the rationale and design for a block-randomized, double-blind, placebo-controlled pilot study. The aim of the pilot study is to explore the creatine uptake in the circulation and tissues following different creatine supplementation dosages.
2. End-Stage Kidney Disease
End-stage kidney disease (ESKD) is one of the world’s leading causes of morbidity and mortality. It is estimated that more than 2.5 million people received kidney replacement therapy worldwide in 2010, and this number is projected to increase to over 5 million by 2030 [
1]. Dialysis is a life-saving treatment, but unfortunately, the health-related quality of life (HRQoL) of dialysis patients is poor and mortality risks are high, as compared with the general population [
2]. Although several potentially modifiable risk factors (e.g., pre-dialysis care and nutritional status) and unmodifiable risk factors (e.g., age and genetics) for excess risk of mortality and poor HRQoL have been identified in dialysis-dependent CKD patients, there is great need for the identification of new, potentially modifiable risk factors [
2,
3,
4,
5,
6,
7]. We hypothesize that creatine deficiency is such a modifiable risk factor, which underlies several important causes for impaired HRQoL in patients with dialysis-dependent chronic kidney disease (CKD), including protein energy wasting (PEW), sarcopenia, fatigue, muscle weakness, depression, cognitive impairment, and increased susceptibility, as well as a higher risk of an adverse course of infectious diseases.
Creatine is a natural nitrogenous organic acid that is integral to energy metabolism, and is crucial for proper cell functioning [
8,
9]. Creatine can be charged to the high-energy product phosphocreatine by creatine kinase and ATP [
4,
10]. In the human body, the majority of creatine (>90%) is present in skeletal muscle, cardiac muscle, smooth muscle, the brain, and in the nervous tissue [
8,
9]. Furthermore, smaller amounts are present in other tissues and cell types, including the kidney, erythrocytes, and leucocytes [
8,
9]. In all these tissues and cells, the phosphocreatine–creatine circuit serves as an energy buffer, facilitating quick transitions in energy requirements [
9]. Importantly, on a daily basis, approximately 1.6–1.7% of the total, mainly intracellular, creatine pool is non-enzymatically degraded to creatinine, which subsequently leaves the cells and is excreted by the kidney as a waste product in urine [
11,
12]. This loss necessitates a continuous replenishment of the total creatine pool, with new creatine to remain in balance [
12].
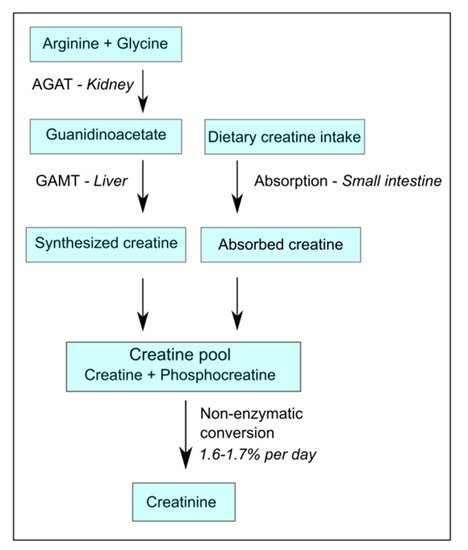
Generally, a common omnivorous diet can provide up to 50% of the daily requirement of creatine replenishment from alimentary sources, like meat, fish, and dairy products [
7,
12]. Coverage of the other 50% requires endogenous synthesis, and the requirements for endogenous synthesis increase if diets become more plant-based. The endogenous synthesis of creatine involves a metabolic pathway, of which the first and rate-limiting step is primarily situated in the kidney [
13], where the enzyme arginine:glycine amidino-transferase (AGAT) converts arginine plus glycine into the creatine precursor guanidinoacetate (GAA) [
14], which is exported from the kidney into circulation [
8,
12]. From there, GAA is taken up into the liver where it is subsequently converted by the enzyme guanidinoacetate methyltransferase (GAMT) into creatine, and is released into the blood stream (
Figure 1) [
4,
8,
12]. Because the rate-limiting step of endogenous creatine synthesis takes place in the kidney, and the capacity for the endogenous synthesis of metabolites by AGAT has been shown to be almost halved after the donation of a kidney [
7], it may be expected that patients with dialysis-dependent CKD with heavily impaired kidney function would also have a virtually absent capacity for endogenous creatine synthesis [
4,
7].
Figure 1. Simplified schematic overview of creatine homeostasis. AGAT-arginine:glycine amidino-transferase; GAMT-guanidinoacetate methyltransferase.
It should also be realized that, apart from decreased or absent endogenous synthesis, creatine losses are likely higher in patients with dialysis-dependent CKD than in healthy subjects. In healthy subjects, creatine losses will mainly be limited to the non-enzymatic conversion of creatine to creatinine, as the majority of creatine is reabsorbed after glomerular filtration [
15,
16,
17]. In patients with dialysis-dependent CKD, creatine losses will be the consequence of the non-enzymatic conversion of creatine to creatinine and—on top of that—of unopposed losses into the dialysate, because the dialysis filter cannot reabsorb creatine after filtration from circulation [
18]. The same holds true for amino acids, including the AGAT substrates glycine and arginine, which are essential for the synthesis of the creatine precursor GAA, and other valuable small water soluble molecules, like GAA itself [
17,
18].
The existing tendency towards a negative creatine balance in chronic dialysis patients is further exacerbated by the current dietary recommendations for patients with CKD, which are increasingly steered towards more plant-based diets [
19,
20]. The reasons for this are that plant-based diets provide lower loads of phosphorus and acid than animal-based diets, which may seem helpful for controlling hyperphosphatemia and acidosis. However, a plant-based diet might put patients with dialysis-dependent CKD at an even higher risk for a negative creatine balance and developing creatine deficiency, because plant-based diets lack naturally derived creatine [
21]. It should be noted that amino acids necessary for the endogenous synthesis of creatine are present in plant-based foods, but because of the absence of kidney function in patients with dialysis-dependent CKD, the enzymatic capacity for the endogenous synthesis of creatine from these amino acids is severely impaired [
4,
7]. This is underscored by the fact that even without dietary recommendations toward a plant-based diet, roughly 43% of the general U.S. population has an average intake of creatine below the recommended daily allowance of 1.0 g of dietary creatine per day [
22].
Together, the poor endogenous synthesis of creatine, unopposed loss of creatine and creatine precursors during dialysis, and low dietary intake of creatine may add up to creatine deficiency in patients with dialysis-dependent CKD. In general, the plasma concentrations of small molecules tend to be higher in hemodialysis patients compared with healthy individuals, because of a lower glomerular filtration rate. However, the fasting plasma creatine and GAA levels are lower in dialysis patients compared with healthy individuals [
18]. This indicates that dialysis-dependent CKD, in comparison with normal subjects, have a generally lower level of creatine and its metabolites. This is supported by the fact that skeletal muscle biopsies in CKD showed significantly low ATP and phosphocreatine levels [
23]. This notion is further supported by studies showing lower phosphocreatine concentrations and a decreased phosphocreatine/ATP energy-charge ratio in the hearts and skeletal muscle of patients on either hemodialysis or peritoneal dialysis treatment, as shown by means of non-invasive in vivo 31P-NMR imaging [
24,
25,
26].
3. Discussion
Creatine supplementation as a nutritional intervention has been extensively studied in several populations, showing promising results [
60,
61,
62]. To date, almost all creatine supplementation studies have been performed with oral supplementation. In dialysis-dependent kidney disease patients, the oral supplementation of creatine is less suitable as it requires dissolving the creatine in large volumes of water, which may negatively affect the fluid balance. In addition, the causes for creatine deficiency in dialysis-dependent kidney disease patients, being impaired endogenous synthesis [
4,
7], losses to the dialysis fluid [
17,
18], and insufficient intake [
19,
20], are chronic in nature and therefore require long-term supplementation of creatine. As many CKD patients are dependent on dialysis for years, sometimes even lifelong, treating creatine deficiency with oral supplementation requires a high level of compliance. In addition, oral supplementation does not prevent losses of creatine to the dialysis fluid. In contrast to oral supplementation, intradialytic supplementation offers the possibility to supplement creatine in a controlled manner, while preventing unopposed losses of creatine to the dialysis fluid, volume overload due to the necessary ingestion of large volumes of water, and potential problems with compliance.
A creatine deficient state is not without consequences. It has become increasingly clear that low creatine levels play an important role in many different causes for impaired HRQoL and have higher mortality rates in hemodialysis patients. For example, a recent study showed that higher plasma creatine concentrations are associated with lower odds of low muscle mass, low protein intake, hypoalbuminemia, and severe fatigue, indicating a potential role for creatine supplementation in hemodialysis patients [
18].
Protein-energy-wasting (PEW), a progressive depletion of protein and energy stores, is highly prevalent in hemodialysis patients (up to 50–75%) and is associated with both increased morbidity and mortality, and impaired quality of life. Potential causes of PEW in patients with dialysis-dependent CKD include reduced protein and energy intake, reduced physical activity, increased catabolism, reduced anabolism, and comorbidities (e.g., diabetes), as well as the dialysis treatment itself, causing, among others, a loss of amino acids to the dialysate [
63,
64]. Additionally, the situation is exacerbated by inflammatory processes, and loss of residual renal function [
63]. These causes often occur simultaneously and exacerbate the general pathological state of dialysis patients.
It has been shown that oral creatine supplementation increases the intramuscular total creatine (creatine plus phosphocreatine) concentrations [
62] and, in parallel, significantly increases muscle mass [
65] and muscle performance for high-intensity and endurance performance [
62,
66]. In addition, there is a growing body of evidence that creatine supplementation, combined with moderate resistance training, can, at least partially, counteract the loss of muscle mass caused by ageing or immobilization [
67,
68].
Furthermore, hemodialysis patients frequently suffer from fatigue [
2,
69]. The burden of fatigue is underscored by the results of a cross-sectional study, in which 94% of the patients would accept more frequent hemodialysis if it would increase their energy level, but only 19% would do so for an increase in survival time of up to 3 years [
70]. It has been hypothesized previously that creatine administration plays an important role in reducing both mental and muscular fatigue by increasing the brain content of phosphocreatine [
71]. Therefore, intradialytic creatine supplementation may benefit hemodialysis patients by potentially attenuating the fatigue often experienced with kidney disease.
Another contributor to increased mortality and impaired HRQoL in hemodialysis patients is cognitive impairment [
72]. Cognitive impairment is associated with lower compliance concerning nutritional restrictions, fluid restrictions, and medication [
73]. The underlying pathogenesis is not fully understood, but it has been indicated that creatine supplementation improves brain health, improves cognition, and is effective at alleviating brain ischemia and hypoxia [
30]. Importantly, creatine has also been shown to alleviate treatment-resistant depression, especially in women [
74,
75,
76], and recent study results indicate a significant negative relationship between dietary creatine intake and depression in a nationally representative adult cohort in the USA, indicating that a low creatine intake may enhance the incidence of depression [
75]. Therefore, intradialytic creatine supplementation may benefit hemodialysis patients by potentially improving the mental complaints often experienced with kidney disease.
Additionally, as creatine has a positive impact on both the development and activation of the innate and adaptive immune response [
31], dialysis patients who are supplemented with creatine may have a lower incidence of infections during the time of chronic dialysis treatments.
Furthermore, as cardiovascular diseases are prominent pathologies in dialysis patients, creatine, with its reported benefit for vascular health, such as alleviating oxidative stress and inflammation [
71,
77,
78], may be helpful in these respects in this vulnerable population.
During hemodialysis treatment, erythrocytes are subject to mechanical and oxidative stress, potentially leading to anemia. Studies suggest that creatine, by its ability to inhibit erythrocyte lipid peroxidation, may contribute to the maintenance of normal cell deformability [
79,
80,
81]. Intradialytic creatine supplementation could potentially lead to reduced losses of erythrocytes, and may therefore reduce the erythropoietin (EPO) requirements of the dialysis patients. The reduced administration of EPO will circumvent the possible EPO-supported progression of tumors and should markedly reduce costs to the healthcare system [
82,
83].
4. Conclusions
Patients with CKD relying on dialysis treatments likely suffer from creatine deficiency due to a decreased endogenous production of creatine and unopposed losses of creatine from the blood into the dialysate. In the current study, we have provided the rationale for intradialytic creatine supplementation and described the study protocol for a pilot study in preparation for a large double-blind, placebo-controlled supplementation trial.
Intradialytic creatine supplementation may help to maintain creatine homeostasis among dialysis-dependent CKD patients, and consequently improve important causes for impaired HRQoL, including protein energy wasting (PEW), fatigue, muscle weakness, depression, and cognitive impairment.
This entry is adapted from the peer-reviewed paper 10.3390/nu13082709