Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Nutrition and rehabilitation are crucial in post-stroke recovery, especially in the elderly. Since stroke is the leading cause of long-term disability, there is a need to promote special, individually tailored nutrition strategies targeting older patients with low motor ability. Chronic stroke survivors have higher risk of developing nutrition-related chronic diseases, such as sarcopenia, anemia, type 2 diabetes mellitus and osteoporosis.
- nutritional supplements
- malnutrition
- neuroprotective diets
1. Introduction
Stroke is one of the most common causes of disability in adults. Currently, despite introducing thrombolysis and thrombectomy treatment, there is still high demand to prevent neuronal death and neurological dysfunction in post-stroke patients. Moreover, patients after stroke have about a 43% higher risk of stroke reoccurrence over 10 years with an increased annual rate of 4% [1].
Therefore, long-term management of risk factors, including proper nutrition, plays a major role in medical care, especially during the rehabilitation process. Prevention of stroke recurrence is mainly based on changes in behavioral strategies and lifestyle factors. The main lifestyle modifiable factors are smoking, diet, obesity, alcohol and physical activity [2].
Generally, effective rehabilitation requires a holistic approach. In most cases, post-stroke rehabilitation begins the day after stroke, but it is a very short program due to the diagnostic and therapeutic process. The intensity of rehabilitation is tailored to patients’ individual needs. However, during the first 2–3 months, when neuroplasticity is very active, the level of patients’ activity is gradually accelerated and focused on functional, cognitive and emotional improvement [3].
Most patients are able to tolerate an increased duration of rehabilitation programs, especially in specialized post-stroke rehabilitation services or units. In different countries, there are different recommendations for the duration of the rehabilitation program [4].
In Poland, the minimum duration of the whole program including physiotherapy, cognition and speech therapy is 150 min daily with a one-day break on Sunday.
One of the most important factors determining post-stroke rehabilitation effectiveness is appropriate nutrition, adjusted to patient’s medical history and increased demands during the complex biochemical processes of brain recovery and high physical and cognitive activity. Moreover, malnutrition is associated with poor clinical and functional outcomes in post-stroke patients, with greater incidence of infections and pressure sores as well as higher lengths of hospitalizations [5]. There are huge differences between reports in the prevalence of malnutrition in patients after a stroke, ranging from 6.1 to even 62%, whilst other studies have reported the rate ranges from 8 to 49%. Routine tests of nutritional status are recommended for all post-stroke patients in order to enhance their potential for recovery and prevent the development of malnutrition during rehabilitation [6][7].
The aim of this entry is to present risk factors for the development of malnutrition and nutrition-related chronic diseases in post-stroke patients (Figure 1); the role of nutrition; and the most effective strategies to prevent malnutrition, including neuroprotective diets and nutritional supplements, in post-stroke rehabilitation. This article focuses on evidence that nutritional status has a correlation with functional recovery during and after rehabilitation. Moreover, a new approach to post-stroke neuroplasticity including treatment with agents from marine sources such as fucoxanthin and tramiprosate as compounds that might be used as potential neuroprotectants with antioxidative and anti-inflammatory properties is introduced.
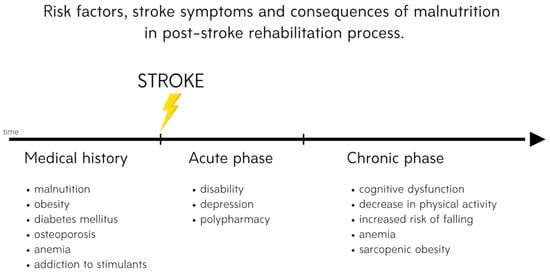
Figure 1. Risk factors, stroke symptoms and consequences of malnutrition in the post-stroke rehabilitation process.
Currently, there is great interest in dietary supplementation to support rehabilitation, alleviate vascular diseases, decrease ischemic brain damage and enhance processes of spontaneous recovery and neuroplasticity. Data from several clinical studies show medical benefits from dietary supplementation not only in functional but also in cognitive and emotional status in post-stroke patients.
2. Risk of Malnutrition after Stroke
Malnutrition is common in patients with neurological conditions, including stroke. The causes of this abnormality are directly related to neurological diseases, such as cognitive functions and disorder of consciousness, neurogenic vomiting, neurogenic dysphagia, depression, motor deficit and gastrointestinal dysfunction [8][9][10].
A significant element of therapeutic and preventive management in central nervous system (CNS) disorders is proper assessment and monitoring of the nutritional status. The reasons for the reduced consumption of nutrients include dysphagia, obstruction or stricture of the esophagus, disability, a general deterioration in condition, increased catabolic processes, digestive disorders and pharmacotherapy [11].
Malnutrition is a well-documented negative prognostic factor both in the general population and in various patient groups. It greatly increases the risk of complications such as pressure ulcers and infections (especially of the respiratory system), electrolyte disturbances, coagulation disorders, anemia, osteoporosis and bradycardia and also reduces the quality of life. Furthermore, it influences the hospitalization time and the number of stays in intensive care units and reduces the effectiveness of rehabilitation [10]. Besides, malnutrition also increases mortality from roughly 30 to 180 days after the ischemic incident. In the Feed Or Ordinary Diet (FOOD) clinical trial it was noted that the odds ratio (OR) of death in the group of post-stroke patients with malnutrition was 2.32 (95% confidence interval (CI) 1.78–3.02) compared to post-stroke patients with normal nutritional status [12].
The most important neurological cause of nutritional disorders is dysphagia, which includes disturbances in the swallowing process at any of its stages: from taking food into the mouth, through keeping it in the buccal cavity, chewing and shaping it, to transporting it from the oral cavity through the throat and esophagus to the stomach. The prevalence of dysphagia in the general population is estimated at 7%; this percentage increases with age, and in the elderly, it reaches 50% [13][14][15]. In patients with CNS disease, the most common cause of dysphagia, the prevalence of dysphagia is estimated to be 50% [16][17].
Neurogenic dysphagia can be caused by disturbances in the coordination of individual swallowing phases, paresis of the muscles involved in the act of swallowing, abnormal muscle tone, disturbance in swallowing–breathing coordination, disturbance of sensation in the mouth or throat, involuntary movements, disturbances in the central control of swallowing or most commonly a combination of these symptoms [18][19][20]. Neurogenic dysphagia is most often associated with neurological deficits that make it difficult to adopt and maintain a proper position while eating and can significantly reduce the critical insight into the symptoms presented. Patients with swallowing disorders also often have sensory disorders, undergo pharmacotherapy that can reduce muscle tone and attention and have problems with dentition. Importantly, a major impediment in the diagnosis of dysphagia is the frequent lack of patients’ consciousness of the problem, which indicates the need for routine assessment of possible swallowing disorders by medical and nursing staff [14][15][16][19].
The most important potentially fatal consequences of dysphagia, regardless of etiology, include dehydration, malnutrition and aspiration to the respiratory system, leading to Mendelson’s syndrome [21][22]. For this reason, early diagnosis of dysphagia and proper nutritional intervention are of key importance for the prognosis of patients with CNS diseases. In all patients with dysphagia, it is extremely important to assess and monitor the nutritional status, including qualitative and quantitative evaluation of consumed food and fluid. This is extremely important because it enables the early identification of patients requiring nutritional support or treatment. In addition, careful oral hygiene, the use of anti-reflux procedures and safe feeding reduce the risk of pneumonia. Hospitalized patients requiring diet modification should have access to properly modified food and fluids [23].
3. Nutrition-Related Chronic Diseases in Post-Stroke Patients
3.1. Osteoporosis
Loss of bone mineral density (BMD) is a common symptom in post-stroke patients. Bone loss begins immediately after stroke and progresses for the first few months. Then, it stabilizes at a lower rate but still does not recede for at least a year [24]. The pathology of post-stroke osteoporosis is complex, as it may involve reduced bone load, reduced mobility, paresis and changes in muscle mass and strength. However, it may also be related to nutritional deficits [24][25]. Long-term hypocalcemia might also contribute to osteoporotic problems. In a recent study by Siotto et al. on calcium levels in post-stroke patients, it was discovered that 26.7% of patients had total calcium levels below the reference range, and it was suggested that serum calcium levels might be correlated with patients’ outcomes during the rehabilitation program. What is more, total protein and albumin were below the reference range in about 77% and 67% of patients. Authors recommend measurements of calcium levels at the beginning and during the rehabilitation program in order to apply appropriate supplementation or dietary program [26]. Osteoporosis not only negatively influences functional recovery, but also is associated with poor cognitive function during the acute and recovery stroke phases [27]. In addition to medications, treatment should also include muscle-strengthening training; resistance training; and appropriate supplementation, which includes vitamin D, folic acid, mecobalamin and calcium [24][25]. It is worth mentioning that only 15.5% of stroke patients receive treatment to prevent bone loss and that pharmacological treatment often prescribed after stroke, such as statins or warfarin, might have a negative influence on bone density [28].
3.2. Anemia
Anemia might be considered as one of the stroke risk factors. In post-stroke patients, it is a predictor of poor outcomes with increased morbidity and mortality [29][30]. Patients with this condition are more likely to have recurrent stroke [31]. In case of anemia, administration of iron is recommended, although excessive iron supplementation might cause side effects. If oral administration of iron is insufficient, it is worth considering the newer intravenous iron complexes (i.e., iron sucrose complexes), which are safer and easily administered [29][32]. In post-stroke patients, anemia should be treated as soon as possible due to the fact that hemoglobin improvement has a positive influence on functional recovery and may reduce the duration of hospitalization [33][34]. When comparing FIM scores between post-stroke patients with and without anemia, the nonanemic group had a significantly higher FIM score improvement and FIM efficiency [33].
3.3. Sarcopenia
Sarcopenia is also a frequent occurrence after stroke with an increased incidence of 14 to 54% [35]. Risk factors for sarcopenia include older age, lack of physical activity and malnutrition, which largely affect patients after stroke. Changes in muscle tissue begin within hours after stroke, and reduction in muscle mass is rapid, which can lead to physical function reduction or even disability [36]. Several factors contribute to sarcopenia occurrence; therefore, it requires an interdisciplinary approach, combining proper rehabilitation and nutritional support. The rehabilitation program should primarily consist of resistance training, but also walking and ADL training, paralyzed limb facilitation and aerobic training to prevent sarcopenic obesity [37]. When it comes to nutrition, studies suggest increased protein intake, high-energy and high-protein meals and leucine-enriched amino acid supplementation [37][38][39].
3.4. Diabetes Mellitus
Diabetes mellitus (DM) is another factor predicting poor outcome with higher mortality after stroke [40][41][42]. In an interesting study by Zhang Y. et al. it was suggested that patients with DM are more likely to suffer from late-onset post-stroke depression [43]. The prevalence of DM is significant; it has been determined that one-third of post-stroke patients have this disease [42]. Both the prevention of stroke among DM patients and post-stroke treatment should primarily include normalizing blood glucose levels [41]. Lifestyle changes such as controlling body weight, minimizing total fat intake (especially saturated fat), consuming a low-carbohydrate diet, consuming low sodium and cholesterol and augmenting fiber intake may also be beneficial [41][44].
This entry is adapted from the peer-reviewed paper 10.3390/nu13082704
References
- Hardie, K.; Hankey, G.J.; Jamrozik, K.; Broadhurst, R.J.; Anderson, C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke 2004, 35, 731–735.
- Han, J.; Mao, W.; Ni, J.; Wu, Y.; Liu, J.; Bai, L.; Shi, M.; Tu, J.; Ning, X.; Wang, J. Rate and Determinants of Recurrence at 1 Year and 5 Years After Stroke in a Low-Income Population in Rural China. Front. Neurol. 2020, 11, 2.
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357.
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169.
- Foley, N.C.; Salter, K.L.; Robertson, J.; Teasell, R.W.; Woodbury, M.G. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke 2009, 40, e66–e74.
- Shen, H.C.; Chen, H.F.; Peng, L.N.; Lin, M.H.; Chen, L.K.; Liang, C.K.; Lo, Y.K.; Hwang, S.J. Impact of nutritional status on long-term functional outcomes of post-acute stroke patients in Taiwan. Arch. Gerontol. Geriatr. 2011, 53, e149–e152.
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocrit. Care 2018, 29, 374–384.
- Chauwa, L.; Appiah, C.A.; Nsiah, K.; Sarfo, F.S. Nutritional risk markers among stroke out-patients at the neurology clinic of a teaching hospital in Ghana. Pan Afr. Med. J. 2020, 37, 258.
- Mullins, N. Nutrition and hydration management among stroke patients in inpatient rehabilitation: A best practice implementation project. JBI Evid. Implement. 2021, 19, 56–67.
- Sato, Y.; Yoshimura, Y.; Abe, T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients 2021, 13, 943.
- Chen, N.; Li, Y.; Fang, J.; Lu, Q.; He, L. Risk factors for malnutrition in stroke patients: A meta-analysis. Clin. Nutr. 2019, 38, 127–135.
- Dennis, M.S.; Lewis, S.C.; Warlow, C.; Collaboration, F.T. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): A multicentre randomised controlled trial. Lancet 2005, 365, 755–763.
- Bramanti, E.; Arcuri, C.; Cecchetti, F.; Cervino, G.; Nucera, R.; Cicciù, M. Dental management in dysphagia syndrome patients with previously acquired brain damages. Dent. Res. J. 2012, 9, 361–367.
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270.
- Roden, D.F.; Altman, K.W. Causes of dysphagia among different age groups: A systematic review of the literature. Otolaryngol. Clin. N. Am. 2013, 46, 965–987.
- Warnecke, T.; Labeit, B.; Schroeder, J.; Reckels, A.; Ahring, S.; Lapa, S.; Claus, I.; Muhle, P.; Suntrup-Krueger, S.; Dziewas, R. Neurogenic Dysphagia: Systematic Review and Proposal of a Classification System. Neurology 2021, 96, e876–e889.
- Lapa, S.; Foerch, C.; Singer, O.C.; Hattingen, E.; Luger, S. Ischemic Lesion Location Based on the ASPECT Score for Risk Assessment of Neurogenic Dysphagia. Dysphagia 2020.
- Singh, A.; Khatri, G.; Handa, K.K. Unusual cause of dysphagia and dysphonia. BMJ Case Rep. 2021, 14, e243060.
- Schumann-Werner, B.; Dogan, I.; Mirzazade, S.; Mall, B.; Overbeck, R.; Honrath, P.; Schulz, J.B.; Reetz, K.; Werner, C.J. Clinical predictors and neural correlates for compromised swallowing safety in Huntington’s Disease. Eur. J. Neurol. 2021.
- Zeng, L.; Song, Y.; Dong, Y.; Wu, Q.; Zhang, L.; Yu, L.; Gao, L.; Shi, Y. Risk Score for Predicting Dysphagia in Patients After Neurosurgery: A Prospective Observational Trial. Front. Neurol. 2021, 12, 605687.
- Kazachkov, M.; Palma, J.A.; Norcliffe-Kaufmann, L.; Bar-Aluma, B.E.; Spalink, C.L.; Barnes, E.P.; Amoroso, N.E.; Balou, S.M.; Bess, S.; Chopra, A.; et al. Respiratory care in familial dysautonomia: Systematic review and expert consensus recommendations. Respir. Med. 2018, 141, 37–46.
- Alty, J.; Robson, J.; Duggan-Carter, P.; Jamieson, S. What to do when people with Parkinson’s disease cannot take their usual oral medications. Pract. Neurol. 2016, 16, 122–128.
- Dziewas, R.; Allescher, H.D.; Aroyo, I.; Bartolome, G.; Beilenhoff, U.; Bohlender, J.; Breitbach-Snowdon, H.; Fheodoroff, K.; Glahn, J.; Heppner, H.J.; et al. Diagnosis and treatment of neurogenic dysphagia—S1 guideline of the German Society of Neurology. Neurol. Res. Pract. 2021, 3, 23.
- Yang, F.Z.; Jehu, D.A.M.; Ouyang, H.; Lam, F.M.H.; Pang, M.Y.C. The impact of stroke on bone properties and muscle-bone relationship: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 211–224.
- Carda, S.; Cisari, C.; Invernizzi, M.; Bevilacqua, M. Osteoporosis after stroke: A review of the causes and potential treatments. Cerebrovasc. Dis. 2009, 28, 191–200.
- Siotto, M.; Germanotta, M.; Santoro, M.; Di Blasi, C.; Loreti, C.; Mastropaolo, S.; Aprile, I. Total Serum Calcium and Recovery after Rehabilitation in Patients with Stroke. Appl. Sci. 2020, 10, 7893.
- Lee, H.Y.; Park, J.H.; Lee, H.; Kim, T.W.; Yoo, S.D. Does Hip Bone Density Differ between Paretic and Non-Paretic Sides in Hemiplegic Stroke Patients? and Its Relationship with Physical Impairment. J. Bone Metab. 2020, 27, 237–246.
- Hsieh, C.Y.; Sung, S.F.; Huang, H.K. Drug treatment strategies for osteoporosis in stroke patients. Expert Opin. Pharm. 2020, 21, 811–821.
- Kaiafa, G.; Savopoulos, C.; Kanellos, I.; Mylonas, K.S.; Tsikalakis, G.; Tegos, T.; Kakaletsis, N.; Hatzitolios, A.I. Anemia and stroke: Where do we stand? Acta Neurol. Scand. 2017, 135, 596–602.
- Li, Z.; Zhou, T.; Li, Y.; Chen, P.; Chen, L. Anemia increases the mortality risk in patients with stroke: A meta-analysis of cohort studies. Sci. Rep. 2016, 6, 26636.
- Milionis, H.; Papavasileiou, V.; Eskandari, A.; D’Ambrogio-Remillard, S.; Ntaios, G.; Michel, P. Anemia on admission predicts short- and long-term outcomes in patients with acute ischemic stroke. Int. J. Stroke 2015, 10, 224–230.
- Bhavi, S.B.; Jaju, P.B. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy. A randomized controlled trial. BMC Pregnancy Childbirth 2017, 17, 137.
- Chan, T.; Ganasekaran, G. The Effect of Anemia on the Functional Outcomes of the Stroke Patients and the Efficiency of their Stroke Rehabilitation. J. Stroke Cereb. Dis. 2015, 24, 1438–1442.
- Yoshimura, Y.; Wakabayashi, H.; Shiraishi, A.; Nagano, F.; Bise, T.; Shimazu, S. Hemoglobin Improvement is Positively Associated with Functional Outcomes in Stroke Patients with Anemia. J. Stroke Cereb. Dis. 2021, 30, 105453.
- Mas, M.F.; González, J.; Frontera, W.R. Stroke and sarcopenia. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 452–460.
- Su, Y.; Yuki, M.; Otsuki, M. Prevalence of stroke-related sarcopenia: A systematic review and meta-analysis. J. Stroke Cereb. Dis. 2020, 29, 105092.
- Nagano, F.; Yoshimura, Y.; Bise, T.; Shimazu, S.; Shiraishi, A. Muscle mass gain is positively associated with functional recovery in patients with sarcopenia after stroke. J. Stroke Cereb. Dis. 2020, 29, 105017.
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6.
- Lathuilière, A.; Mareschal, J.; Graf, C.E. How to Prevent Loss of Muscle Mass and Strength among Older People in Neuro-Rehabilitation? Nutrients 2019, 11, 881.
- Hill, M.D. Stroke and diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 167–174.
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am. J. Med. Sci. 2016, 351, 380–386.
- Lau, L.H.; Lew, J.; Borschmann, K.; Thijs, V.; Ekinci, E.I. Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J. Diabetes Investig. 2019, 10, 780–792.
- Zhang, Y.; He, J.R.; Liang, H.B.; Lu, W.J.; Yang, G.Y.; Liu, J.R.; Zeng, L.L. Diabetes mellitus is associated with late-onset post-stroke depression. J. Affect. Disord. 2017, 221, 222–226.
- Gaillard, T.; Miller, E. Guidelines for Stroke Survivors with Diabetes Mellitus. Stroke 2018, 49, e215–e217.
This entry is offline, you can click here to edit this entry!
