2. Carotenoids and Risk of AMD (Observational Studies)
Currently, dietary modifications remain the mainstay of therapeutic strategies, to potentially delay or prevent both the development and progression of AMD. The Age-Related Eye Disease Study (AREDS) is considered to be among the most influential large-scale clinical trials highlighting the relationship between dietary antioxidants and the risk of AMD progression [
181]. Reports indicate that regular consumption of the AREDS micronutrient formula (containing vitamin C, vitamin E, beta-carotene, and zinc) offered modest benefits, reducing the risk of late AMD progression by up to 25% during a five-year follow-up with at risk patients [
181]. In aging retinae, it is believed that the depletion of endogenous and exogenous antioxidants represents a critical driver in exacerbating neurodegenerative mechanisms. In fact, there is substantial evidence in favor of the neuroprotective association, between greater dietary consumption of carotenoid nutraceuticals, increased lutein and zeaxanthin concentrations in serum, and AMD prevention. A summary of these observational epidemiology studies is outlined in
Table 3.
Table 3. Epidemiology studies on AMD risk associated with dietary intake and/or serum levels of lutein and zeaxanthin.
| Authors (Year) |
Study Name |
Participants |
Follow-Up |
Assessment of L/Z |
Results |
| Seddon (1994) [197] |
EDCCS |
356 AMD patients, 520 controls in USA; aged 55–80 years |
- |
Dietary L/Z |
Highest quintile of L/Z intake, such as spinach and collard greens, strongly associated with reduced risk of late AMD |
| VandenLangenberg (1998) [201] |
Beaver Dam Eye Study |
1709 individuals in USA; aged 43–84 years |
5 years |
Dietary L/Z |
No significant association reported between incident large drusen and dietary intake |
| Mares-Perlman (2001) [194] |
NHANES III |
8596 individuals in USA; aged ≥40 years |
- |
Dietary L/Z |
Significantly lower risk of pigmentary abnormalities and late AMD in highest L/Z quintiles |
| Snellen (2002) [198] |
- |
72 AMD patients, 66 controls in Netherlands; aged ≥60 years |
- |
Dietary L/Z |
Low dietary intake significantly associated with higher risk of neovascular AMD |
| Cho (2004) [188] |
NHS and HPFS |
77,562 female and 40,866 male health professionals in USA; aged ≥50 years |
18 years;
12 years |
Dietary L/Z |
No significant association between relative risk of age-related maculopathy and vegetable consumption or carotenoid intake |
| Van Leeuwen (2005) [200] |
The Rotterdam Study |
4170 individuals in Netherlands; aged 55–95 years |
8 years |
Dietary L/Z |
No significant association reported between dietary L/Z intake and incident AMD |
| Moeller (2006) [196] |
CAREDS |
1787 women in USA; aged 50–79 years |
7 years |
Dietary L/Z |
Protective association among adult women (<75 years) with stable dietary intake and no history of chronic disease |
| AREDS Research Group (2007) [51] |
AREDS |
4159 AREDS participants in USA; aged 60–80 years |
- |
Dietary L/Z |
Top quintile of dietary L/Z inversely associated with large drusen, neovascular AMD, and geographic atrophy |
| Tan (2008) [199] |
Blue Mountains Eye Study |
2454 individuals in Australia; aged 49–93 years |
10.5 years |
Dietary L/Z |
Greater intake of L/Z saw reduced risk developing soft/reticular drusen and neovascular AMD progression |
| Cho (2008) [187] |
NHS and HPFS |
71,494 female and 41,564 male health professionals in USA; aged 50–79 years |
18 years;
16 years |
Dietary L/Z |
A non-linear, inverse association seen among top quintiles of L/Z intake and neovascular AMD in both cohorts |
| Ho (2011) [16] |
The Rotterdam Study |
2167 individuals in Netherlands; aged ≥55 years |
8 years |
Dietary L/Z |
Top tertile of L/Z intake significantly reduced incident early AMD in those with greater genetic risk |
| Wu (2015) [202] |
NHS and HPFS |
63,443 female and 38,603 male health professionals in USA; aged 50–90 years |
26 years;
24 years |
Dietary L/Z |
Greater consumption of cooked spinach (0.5 cup, >1 serving/wk) inversely associated with intermediate AMD. Late AMD risk significantly lowered by up to 40% with higher L/Z intake |
| Arslan (2019) [186] |
- |
100 AMD patients, 100 controls in Turkey; aged ≥50 years |
- |
Dietary L/Z |
Non-significant association observed between serum L/Z |
| EDCCS Group (1993) [191] |
EDCCS |
421 AMD patients, 615 controls in USA; aged 55–80 years |
- |
Serum L/Z |
Protective association with greater serum L/Z levels and risk of neovascular AMD |
| Mares-Perlman (1995) [193] |
Beaver Dam Eye Study |
167 AMD patients, 167 controls in USA; aged 43–84 years |
- |
Serum L/Z |
No overall association between serum L/Z and risk of late AMD |
| Gale (2003) [192] |
- |
380 individuals in Sheffield, United Kingdom; aged ≥60 years |
- |
Serum L/Z |
Serum Z strongly associated with risk of incident early and late AMD |
| Dasch (2005) [189] |
MARS |
586 AMD patients, 182 controls in Germany; aged 59–82 years |
- |
Serum L/Z |
No significant association reported between serum L/Z levels |
| Delcourt (2006) [190] |
POLA |
640 individuals in Sète, France; aged ≥60 years |
- |
Serum L/Z |
Highest combined serum L/Z has significantly reduced risk |
| Michikawa (2009) [195] |
- |
722 individuals in Karabuchi Town of Takasaki City, Japan; aged ≥65 years |
- |
Serum L/Z |
No significant association found between serum L/Z |
| Zhou (2011) [109] |
- |
174 AMD patients, 89 controls in China; aged 50–88 years |
- |
Serum L/Z |
Significant inverse association between serum Z and neovascular AMD |
It is evident that the relationship between dietary behaviors, lifestyle choices, and the risk of AMD is complex and multifaceted, wherein unhealthy lifestyles often carry an increased risk of consequent disease [
232]. Among older adults, early lifestyle modifications for the systemic management of metabolic syndrome is vital for slowing disease progression, as it also represents another risk factor [
65,
233]. Additionally, the National Eye Institute encourages greater consumption of leafy green vegetables to lower AMD risk [
1]. Multiple longitudinal cohort studies have demonstrated greater consumption of foods such as spinach, kale, and collard greens on a regular basis carry significant protection against incident late AMD [
51,
187,
194,
196,
197,
199]. In fact, these green leafy vegetables, from the cruciferous
Brassica oleracea cabbage species, are recognized as an excellent sources of xanthophyll carotenoids, lutein and zeaxanthin [
197,
234,
235,
236,
237]. A meta-analysis by Ma et al. found that individuals with the highest levels of carotenoid intake saw a significant reduction in the risk of late AMD (pooled relative risk (RR) = 0.74; 95% confidence interval (CI): 0.57–0.97) and saw a 32% risk reduction for neovascular AMD (RR = 0.68; 95% CI: 0.51–0.92) [
238]. Using data from the AREDS cohort, the calculated odds ratios (OR) from one case-control study seemed to corroborate these findings, when comparing the highest versus lowest quintiles of carotenoid intake. Greater dietary consumption of lutein and zeaxanthin offered an enhanced protection against neovascular AMD (OR = 0.65; 95% CI: 0.45–0.93), geographic atrophy (OR = 0.45; 95% CI: 0.24–0.86), and large, or extensive, intermediate drusen (OR = 0.73; 95% CI: 0.56–0.96) [
51]. Evidence from a large cohort of studies largely seemed to implicate that the protective benefits of greater carotenoid intake may be confined to late AMD. However, one report, from the population-based Rotterdam Study, found that higher dietary antioxidants (including lutein and zeaxanthin) may significantly attenuate early AMD incidence conferred by genetic risk variants [
16,
25]. These results are encouraging, given that the AMD risk, among carriers of the
CFH Y402H variant, increased by up to 11-fold, and those with the ARMS2 (
LOC387715 A69S) variant carried up to 15-times greater risk [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25]. One school of thought suggests that xanthophyll carotenoids afford synergistic neuroprotection against these risk alleles by limiting the overactivation of the complement system concomitant with mitochondriotropic augmentation, respectively [
239,
240,
241,
242]. Furthermore, these findings highlight the importance of stable dietary behaviors, involving the frequent consumption of nutraceuticals rich in lutein and zeaxanthin, for the management of established AMD.
Observational studies investigating the relationship between the serum levels of macular carotenoids provide some evidence of the protective benefits against age-related maculopathy [
109,
190,
191,
192]. The 1993 Eye Disease Case-Control Study first reported that greater levels of lutein and zeaxanthin in serum were inversely associated with the risk of neovascular AMD [
191]. However, a 1995 analysis from the Beaver Dam Eye Study was unable to reproduce these findings and did not find serum levels to correlate with late AMD prevention [
193]. Variation among these initial reports may be explained, at least in part, by differences in the ethnogeography of the sample, and sample size, whereby influencing the interpretability of these results. Moreover, serum analyses from population-based cohorts in the United Kingdom, France, and China seemed to corroborate the protective association with systemic increases of lutein and zeaxanthin concentrations in circulation [
109,
190,
192]. Surprisingly, two of these studies illustrated that the serum levels of zeaxanthin were strongly associated with the risk of incident AMD (both early and late AMD) [
109,
192]. It is well known that differential dietary habits have significant implications on their absorption from food matrices, as well as subsequent concentrations within the plasma [
235,
243,
244,
245,
246,
247,
248,
249,
250], and may, therefore, account for some of the inconsistency among reports.
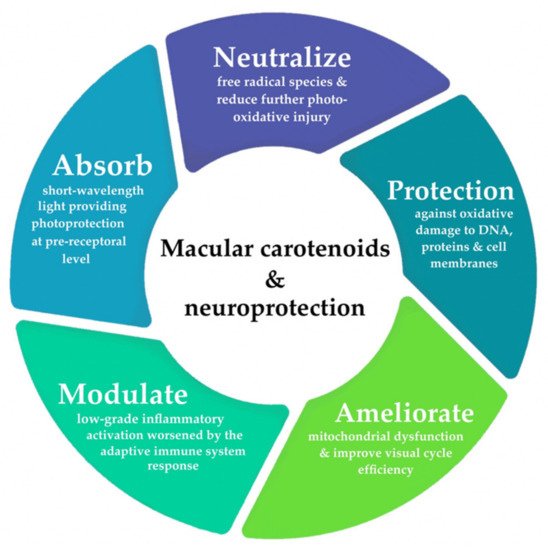
It is important to note that similar biological mechanisms, which greatly reduce the bioavailability of lutein and zeaxanthin, are also involved with established AMD risk factors. The cumulative effect of compromised antioxidant capacity, in consequence of prolonged oxidative injury, is thought to create an overwhelming, neurodegenerative environment. Mitochondrial dysfunction and photo-oxidation are known to trigger the proliferation of premature cellular senescence in RPE, which subsequently triggers the pathogenic cascade of AMD development [
32,
38,
49,
239,
240,
241,
242,
251,
252,
253,
254,
255,
256,
257,
258,
259,
260,
261,
262]. Moreover, in diabetic retinopathy, the underlying causes of metabolic syndrome have been shown to substantially compromise the assimilation and transport of dietary carotenoids [
30,
43,
263,
264,
265,
266,
267,
268,
269,
270,
271,
272,
273,
274,
275,
276,
277,
278,
279,
280,
281,
282]. Metabolic perturbations, such as obesity, insulin resistance, and chronic hyperglycemia promote atherogenic metabolic imbalance, which further contributes to macular pigment depletion [
102,
250,
276,
277,
278,
279,
280,
281,
282,
283,
284,
285]. Therefore, low MPOD levels likely represent an essential factor in AMD development.
Conversely, some observational studies were unable to confirm these benefits [
186,
187,
189,
193,
195,
201]. For instance, the Beaver Dam Eye Study found that neither dietary intake nor serum carotenoid levels were significantly correlated with AMD [
193,
201], while the Muenster Aging and Retina Study (MARS), in Germany, also observed a non-statistically significant association between plasma concentrations and age-related maculopathy [
189]. Inconsistency among large-scale cohort studies may be attributed, at least in part, to the persistent challenges of investigating insidious neurodegenerative conditions, such as AMD. Etiologically relevant exposures involve a combination of lifestyle choices and dietary habits, culminating over years or decades before the date of diagnosis, as clinical manifestations often present themselves only after incurring extensive damage to the retina. Thirteen out of twenty (13/20) reports from several large-scale epidemiological studies demonstrated the effects of xanthophylls in protecting against the progression of AMD [
16,
51,
109,
187,
190,
191,
192,
194,
196,
197,
198,
199,
202]. Thus, it is appropriate to summarize that the majority of the observational studies discussed herein advocate the benefits of xanthophyll carotenoids in AMD.
3. Carotenoids in the Management of AMD (Interventional Studies)
Given the effectiveness of the AREDS supplement in slowing the course of AMD progression, randomized clinical trials have investigated the efficacy of carotenoid vitamin therapy, supplemented with or without additional antioxidants and micronutrients. It is important to note that the original AREDS formulation did not include xanthophyll carotenoids (i.e., lutein or zeaxanthin) [
65]; instead, it contained β-carotene, which belongs to the subclass of provitamin A carotenes [
286]. However, in response to the discovery that β-carotene may correlate with a greater risk of developing lung cancer among cigarette smokers [
287,
288], the Age-Related Eye Disease Study 2 (AREDS2) modified the original formula by removing β-carotene and substituting with lutein and zeaxanthin [
50,
289]. Primary analysis from the AREDS2 trial suggested xanthophyll supplementation did not offer further benefits against the rate of AMD progression, in comparison to the original AREDS formula [
50]. However, secondary analysis showed that lutein and zeaxanthin supplementation significantly improved protection against late AMD (hazard ratio (HR): 0.82; 95% CI: 0.69–0.96) and particularly against neovascular AMD (HR: 0.78; 95% CI: 0.64–0.94), when substituted for β-carotene in the AREDS formulation [
203]. The risk reduction was most significant among those with intermediate AMD lesions (bilateral large drusen) at baseline; direct comparisons showed HRs of 0.76 (95% CI: 0.61–0.96;
p = 0.02) for developing late AMD and 0.65 (95% CI: 0.49–0.85;
p = 0.002) for neovascular AMD [
203]. From these reports, the AREDS2 suggests that supplementation with lutein and zeaxanthin could offer enhanced protection and are well-suited for therapeutic management using nutraceuticals in patients with established AMD, particularly in lieu of β-carotene.
Numerous randomized clinical trials have been extremely consistent in demonstrating that xanthophyll carotenoid supplementation can greatly improve their concentrations in serum [
50,
115,
119,
204,
205,
206,
207,
213,
217,
219,
221,
222,
227,
229,
231] and within the retinal tissue (i.e., MPOD) among patients with AMD [
56,
115,
117,
119,
120,
204,
205,
206,
210,
213,
214,
215,
216,
222,
225,
226,
228,
230,
231]. A summary of these randomized clinical trials is outlined in
Table 4 and
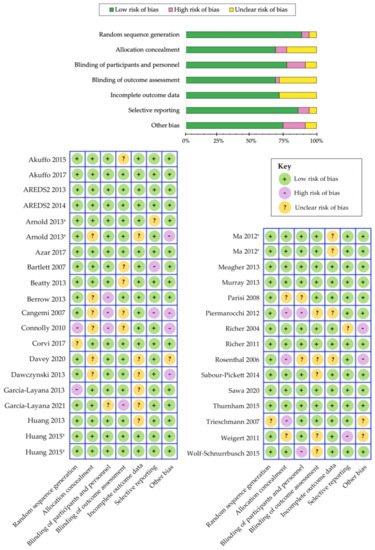
Table 5. We have evaluated the risk of bias among the various randomized controlled trials using the Cochrane Collaboration’s tool [
185] and a summary is shown in
Figure 3. Overall, we determined that the risk of bias was low among the randomized controlled trials that evaluated the benefits of carotenoid vitamin therapy in AMD. A meta-analysis by Ma et al., comparing nine carotenoid interventional trials, revealed a dose-response relationship that was positively correlated with increased MPOD levels and changes in plasma concentrations, following supplementation with lutein, zeaxanthin, and/or
meso-zeaxanthin [
56]. Stratified analysis demonstrated that the augmentation of the macular pigment was most effective when supplementing with all three xanthophyll carotenoids during trials lasting longer than 12 months [
56]. A stronger effect was also noted for studies containing higher doses of these carotenoids (per daily serving). Furthermore, reports from similar clinical trials seem to corroborate these findings, wherein treatment with macular carotenoids offered significant improvements to MPOD levels in eyes with AMD, upon measurement with both subjective and objective techniques [
115,
117,
205,
210,
213,
214,
226,
228,
230,
231]. Consistency among these results is highly significant because changes in the macular pigment measured, in response to carotenoid vitamin therapy, substantiate the role of MPOD status in representing a pharmacodynamic/response biomarker in the context of AMD.
Figure 3. Cochrane Collaboration’s tool for assessing risk of bias in randomized controlled trials [
185]. Separate publications are indicated with a symbol (†) next to author name.
Table 4. Characteristics of the interventional studies reporting on serum carotenoid levels in AMD.
| Authors (Year) |
Study |
Participants |
Duration |
Interventions |
Serum |
Main Findings |
| Rosenthal (2006) [227] |
- |
30 patients with intermediate or late AMD; aged 60–91 years in USA |
6 months |
2.5 mg L; 5 mg L; 10 mg L |
L and Z |
Mean serum concentrations rose in each dosage group by 2-fold, 2.9-fold and 4-fold, respectively (p < 0.001 for all) |
| Trieschmann (2007) [119] |
LUNA |
100 patients with AMD; aged (71.5 ± 7.1) years in Germany |
6 months |
12 mg L and 1 mg Z * (multivitamin); placebo |
L and Z |
Substantial increase in L (4-fold rise; p < 0.001) and Z (p = 0.007) concentrations |
| Connolly (2010) [213] |
MOST |
5 patients with early AMD; aged (72.0 ± 11.0) years in Ireland |
2 months |
7.3 mg MZ, 3.7 mg L and 0.8 mg Z |
L, Z and MZ |
Significant time effect between rise in all three carotenoid serum levels (p < 0.003 for all) |
| AREDS2 Research Group (2013) [50] |
AREDS2 |
4203 patients with intermediate or late AMD; aged (73.1 ± 7.7) years in USA |
5 years |
10 mg L and 2 mg Z * (multivitamin); 10 mg L, 2 mg Z and omega-3 fatty acids * (multivitamin); “placebo” |
L and Z |
Total serum L + Z levels increased by 190% to 210% from baseline (p < 0.001) |
| Arnold (2013) [206] |
- |
20 patients with atrophic AMD; aged (66.0 ± 8.0) years in Germany |
4 weeks |
10 mg L and 3 mg Z, in oleaginous kale extract |
L and Z |
Statistically significant rise in serum L and serum Z after 4 weeks (p < 0.001 for both) |
| Arnold (2013) [207] |
LUTEGA |
172 patients with atrophic AMD; aged (69.0 ± 10.0) years in Germany |
12 months |
10 mg L and 1 mg Z * (multivitamin); 20 mg L and 2 mg Z * (multivitamin); placebo |
L and Z |
Beneficial alterations seen in both treatment groups (p < 0.05) after one-month and values remained elevated until trial completion |
| Huang (2013) [219] |
- |
108 patients with early AMD; aged 50–81 years in China |
48 weeks |
10 mg L; 20 mg L; 10 mg L and 10 mg Z; placebo |
L and Z |
Greater increase in serum L and Z with high-dose L (6.23-fold) and L + Z formula (3.11-fold), respectively (p < 0.001 for both) |
| Meagher (2013) [221] |
- |
27 patients with early AMD; aged (66.0 ± 9.0) years in Ireland |
8 weeks |
20 mg L, 2 mg Z and 0.3 mg MZ; 10 mg L, 2 mg Z
and 10 mg MZ; 3 mg L, 2 mg Z and 17 mg MZ |
L, Z and MZ |
Serum L and Z increased only with higher-dose L (Groups 1 and 2; p < 0.001) while serum MZ increased in all three groups (p < 0.01 for all) |
| Murray (2013) [222] |
CLEAR |
72 patients with early AMD; aged (70.5 ± 8.7) years in United Kingdom |
12 months |
10 mg L; placebo |
L |
Marked increase in serum L (p < 0.001) compared to placebo control |
| Akuffo (2015) [205] |
MOST |
52 patients with early AMD; aged (66.0 ± 8.0) years in Ireland |
3 years |
20 mg L and 2 mg Z; 10 mg L, 2 mg Z and 10 mg MZ; 3 mg L, 2 mg Z and 17 mg MZ |
L, Z and MZ |
Statistically significant time x treatment effect revealed for changes serum L and MZ (p < 0.05 for both) concentrations |
| Huang (2015) [115] |
- |
112 patients with early AMD; aged (69.1 ± 7.4) years in China |
24 months |
10 mg L; 20 mg L; 10 mg L and 10 mg Z; placebo |
L and Z |
Highly significant time x treatment interaction observed for both serum L and Z (p < 0.001 for both) |
| Wolf-Schnurrbusch (2015) [231] |
- |
79 patients with early/intermediate AMD; aged 55–88 years in Switzerland |
6 months |
10 mg L and 1 mg Z * (multivitamin); 10 mg L, 1 mg Z and omega-3 fatty acids * (multivitamin); placebo |
L and Z |
Increases in serum L and Z (p < 0.05) only reported in Group 1 (carotenoid treatment without omega-3 fatty acids in formula) |
| Akuffo (2017) [204] |
CREST |
121 patients with early/intermediate AMD; aged (64.7 ± 9.0) years in Ireland |
24 months |
10 mg L and 2 mg Z * (multivitamin); 10 mg L and 10 mg Z * (multivitamin); placebo |
L, Z and MZ |
Remarkable increase in all three serum concentrations (p < 0.0005); time x group interaction effect only for serum Z and MZ (p < 0.005 for both) |
| Sawa (2020) [229] |
Sakai Lutein Study |
39 patients with neovascular AMD; aged (70.7 ± 5.3) years in Japan |
6 months |
20 mg L and 3 mg Z (beeswax capsule); 20 mg L and 3 mg Z (glycerol capsule) |
L |
Serum L increased in both treatment groups at 3- and 6 months (p < 0.01 for both) |
| García-Layana (2021) [217] |
- |
109 patients with neovascular AMD; aged (77.1 ± 7.6) years in Spain |
12 months |
10 mg L and 2.6 mg Z * (multivitamin); original AREDS formula (no L/Z) |
L and Z |
Substantial increase in serum L and Z (p < 0.001 for both) with a large effect size after 12 months (Cohen’s d of ≥0.80 for both) |
Table 5. A summary of the eligible randomized clinical trials reporting on AMD.
| Authors (Year) |
Study |
Participants |
Duration |
Interventions |
MPOD |
Main Findings |
| Richer (2004) [225] |
LAST |
90 patients with atrophic AMD; aged (74.7 ± 7.4) years in USA |
12 months |
10 mg L; 10 mg L * (multivitamin); placebo |
HFP |
Significant benefit in MPOD (p < 0.001), BCVA (p < 0.01) and CS at low/middle spatial frequencies (p < 0.05 for all) |
| Bartlett (2007) [209] |
- |
25 patients with atrophic AMD; aged (69.2 ± 7.8) years in USA |
9 months |
6 mg L; placebo |
- |
Non-significant trend towards improvement in CS reported |
| Cangemi (2007) [212] |
TOZAL |
37 patients with atrophic AMD; aged (76.3 ± 7.8) years in USA |
6 months |
8 mg L and 0.4 mg Z * (multivitamin) |
- |
Modest improvements observed in BCVA (p = 0.045) |
| Trieschmann (2007) [119] |
LUNA |
100 patients with AMD; aged (71.5 ± 7.1) years in Germany |
6 months |
12 mg L and 1 mg Z * (multivitamin);placebo |
Fundus AFI |
Mean increase of +15.9% in MPOD measured at 0.5° eccentricity (p < 0.001) compared to control |
| Parisi (2008) [223] |
CARMIS |
27 patients with atrophic AMD; aged (65.5 ± 5.1) years in Italy |
12 months |
10 mg L + 1 mg Z * (multivitamin); placebo |
- |
Enhanced improvement in central retinal function measures on mfERG (ring 1 and ring 2; p < 0.01 for both) |
| Connolly (2010) [213] |
MOST |
5 patients with early AMD; aged (72.0 ± 11.0) years in Ireland |
2 months |
7.3 mg MZ, 3.7 mg L and 0.8 mg Z |
cHFP |
Significant increase in MPOD measured at 0.25° and 1° eccentricity with respect to time (p < 0.05 for all) |
| Richer (2011) [226] |
ZVF |
60 patients with early/intermediate AMD; aged (74.9 ± 10.0) years in USA |
12 months |
8 mg Z; 8 mg and 9 mg L; 9 mg L |
HFP |
Central (1°) MPOD increased in all three groups (p < 0.03 for all); significant improvement in measures of foveal vision greater in Z-only group, while benefits in parafoveal vision were greater in L-only group |
| Weigert (2011) [120] |
LISA |
126 patients with early/intermediate AMD; aged (71.6 ± 8.6) years in Austria |
6 months |
20 mg L for 3 months, then 10 mg L for 3 months; placebo |
Reflectometry |
Average increase of +27.9% in MPOD (p < 0.001); trend toward improvement in BCVA did not reach statistical significance |
| Ma (2012) [117] |
- |
108 patients with early AMD; aged 50–81 years in China |
48 weeks |
10 mg L; 20 mg L; 10 mg L and 10 mg Z; placebo |
Fundus AFI |
Significant dose-response effect with increased MPOD (p < 0.01) positively related to benefits in CS (p < 0.05) and central retina function on mfERG (p < 0.01) |
| Piermarocchi (2012) [224] |
CARMIS |
145 patients with atrophic AMD; aged (72.5 ± 7.0) years in Italy |
24 months |
10 mg L + 1 mg Z * (multivitamin); placebo |
- |
Reported significant benefits in BCVA and CS at 6-, 12-, and 24 months (p < 0.01 for all) compared to placebo |
| AREDS2 Research Group (2013) [50] |
AREDS2 |
4203 patients with intermediate or late AMD; aged (73.1 ± 7.7) years in USA |
5 years |
10 mg L and 2 mg Z * (multivitamin); 10 mg L, 2 mg Z and omega-3 fatty acids * (multivitamin); “placebo” |
- |
Reduced hazard ratios of 0.82 (95% CI:0.69–0.96; p = 0.02) for late AMD and 0.76 (95% CI: 0.64–0.94; p = 0.01) for neovascular AMD compared to β-carotene in formulation † |
| Arnold (2013) [206] |
- |
20 patients with atrophic AMD; aged (66.0 ± 8.0) years in Germany |
4 weeks |
10 mg L and 3 mg Z, in oleaginous kale extract |
Reflectometry |
Enhanced augmentation of macular pigment parameters including volume, area and maxOD (p < 0.001 for all) |
| Beatty (2013) [210] |
CARMA |
433 patients with early AMD; aged (73.9 ± 8.1) years in Ireland |
12 months |
12 mg L and 0.6 mg Z * (multivitamin);
placebo |
Raman spectroscopy |
Statistically significant increase in MPOD with a positive linear trend during trial period (p < 0.01 for all) |
| Berrow (2013) [211] |
- |
14 patients with early AMD; aged (67.6 ± 8.4) years in UK |
40 weeks |
12 mg L and 0.6 mg Z * (multivitamin);
placebo |
- |
Remarkable benefits in mfERG N1P1 response amplitude densities in ring 3 (p = 0.027); no differential changes observed in BCVA and CS |
| Dawczynski (2013) [215] |
LUTEGA |
145 patients with atrophic AMD; aged (70.0 ± 10.0) years in Germany |
12 months |
10 mg L and 1 mg Z * (multivitamin); 20 mg L and 2 mg Z * (multivitamin); placebo |
Reflectometry |
Significant improvements observed for MPOD parameters (volume, area, maxOD and mean OD) and BCVA (p < 0.001 for all) in both treatment groups |
| García-Layana (2013) [216] |
- |
44 patients with early AMD; aged (68.5 ± 8.5) years in Spain |
12 months |
12 mg L and 0.6 mg Z * (multivitamin);
placebo |
HFP |
Considerable rise in MPOD (+0.162 ODU; p < 0.01); however, no significant changes seen in BCVA and CS |
| Murray (2013) [222] |
CLEAR |
72 patients with early AMD; aged (70.5 ± 8.7) years in United Kingdom |
12 months |
10 mg L; placebo |
cHFP |
Highly statistically significant increase in MPOD (+39.5%; p < 0.001) when compared to placebo |
| Sabour-Pickett (2014) [228] |
MOST |
52 patients with early AMD; aged (66.0 ± 8.0) years in Ireland |
12 months |
20 mg L and 2 mg Z; 10 mg L, 2 mg Z and 10 mg MZ; 3 mg L, 2 mg Z and 17 mg MZ |
cHFP |
Robust improvements in MPOD spatial profile observed in those supplemented all three carotenoids in formulation (Group 2, p < 0.005; Group 3, p < 0.05) |
| Akuffo (2015) [205] |
MOST |
52 patients with early AMD; aged (66.0 ± 8.0) years in Ireland |
3 years |
20 mg L and 2 mg Z; 10 mg L, 2 mg Z and 10 mg MZ; 3 mg L, 2 mg Z and 17 mg MZ |
cHFP |
Clinically meaningful CS benefits were seen in all three groups (p < 0.05 for all): Group 1 (15.15 cpd), Group 2 (1.2-, 6- and 9.6 cpd) and Group 3 (6-, 9.6- and 15.15 cpd) |
| Huang (2015) [115] |
- |
112 patients with early AMD; aged (69.1 ± 7.4) years in China |
24 months |
10 mg L; 20 mg L; 10 mg L and 10 mg Z; placebo |
Fundus AFI |
Highly significant time x treatment interaction (p < 0.001) between changes in MPOD and central retinal function improvements (mfERG and MRS; p < 0.05 for both) |
| Thurnham (2015) [230] |
- |
32 patients with early AMD; aged (66.0 ± 9.0) years in Ireland |
8 weeks |
20 mg L, 2 mg Z and 0.3 mg MZ; 10 mg L, 2 mg Z and 10 mg MZ; 3 mg L, 2 mg Z and 17 mg MZ |
cHFP |
Significant increase in all three groups (p < 0.05); Group 2 formulation (10 mg L, 2 mg Z and 10 mg MZ) may offer greater improvement to macular pigment spatial profile |
| Wolf-Schnurrbusch (2015) [231] |
- |
79 patients with early/intermediate AMD; aged between 55–88 years in Switzerland |
6 months |
10 mg L and 1 mg Z * (multivitamin); 10 mg L, 1 mg Z and omega-3 fatty acids * (multivitamin); placebo |
Fundus AFI |
Demonstrable benefits in MPOD (p < 0.005) and CS scores (p < 0.01) observed in Group 1 only (carotenoid treatment without omega-3 fatty acids in formulation) |
| Akuffo (2017) [204] |
CREST |
121 patients with early/intermediate AMD; aged (64.7 ± 9.0) years in Ireland |
24 months |
10 mg L, 2 mg Z and 10 mg MZ * (AREDS2 multivitamin); 10 mg L and 10 mg Z * (AREDS2 multivitamin) |
cHFP |
Augmentation of MPOD (p < 0.001) with clinically meaningful benefits in visual function (CS and GD under mesopic and photopic conditions, photostress recovery, and mean/max reading speed; p < 0.05 for all) |
| Azar (2017) [208] |
- |
79 patients with neovascular AMD; aged (75.3 ± 7.6) years in France |
8 months |
5 mg L and 1 mg Z * (multivitamin);
placebo |
Reflectometry |
Non-significant trend toward greater MPOD levels reported in patients with neovascular AMD |
| Corvi (2017) [214] |
- |
39 patients with early AMD; aged (78.0 ± 6.5) years in France |
3 months |
10 mg L and 2 mg Z * (multivitamin) |
HFP |
Significant rise in MPOD only in eyes with reticular pseudodrusen (n = 19; p = 0.002) after 3 months |
| Davey (2020) [53] |
- |
56 patients with subclinical AMD; aged (68.4 ± 5.3) years in USA |
6 months |
15 mg L, 10 mg MZ and 2 mg Z * (Lumega-Z); 10 mg L and 2 mg Z * (AREDS-2 multivitamin); placebo |
HFP |
Statistically significant CS improvements for Lumega-Z group only (p < 0.001) with a positive linear trend with treatment time (p < 0.001) |
| Sawa (2020) [229] |
Sakai Lutein Study |
39 patients with neovascular AMD; aged (70.7 ± 5.3) years in Japan |
6 months |
20 mg L and 3 mg Z (beeswax capsule); 20 mg L and 3 mg Z (glycerol capsule) |
Fundus AFI |
No significant changes were observed in CS, mesopic glare or MPOD in both treatment groups |
Based on the functional relationship between the macular pigments and sharp central vision, alterations in MPOD status have been postulated as a surrogate of visual performance in both healthy and diseased states [
42]. In fact, a recent systematic review and meta-analysis by Johnson et al. found that MPOD was significantly correlated with visual function outcomes, including visual acuity, contrast sensitivity, photostress recovery, glare discomfort/disability, and dark adaptation in adults with healthy eyes [
54]. Prior reports have also shown that improvements in visual function are positively associated with greater macular pigment levels [
54,
55,
290,
291,
292,
293,
294,
295,
296,
297,
298,
299], which can also be achieved through carotenoid supplementation in healthy eyes [
55,
57,
58,
111,
112,
114,
116,
298,
299,
300,
301,
302,
303,
304,
305,
306]. Thus, evidence from AMD trials, wherein carotenoid vitamin therapy is found to enrich MPOD concentrations concomitant with improvements in visual outcome measures, may be clinically beneficial for older adults and those with AMD, as it will likely render daily activities, such as reading or watching television, safer and easier [
55,
292].
In a meta-analysis by Liu et al. comparing data from several randomized, double-blind, placebo-controlled trials found that carotenoid supplementation resulted in significant improvements in best-corrected visual acuity (BCVA) and contrast sensitivity (CS) at all spatial frequencies in a dose-response relationship [
307]. Correlation analysis revealed a linear association between the augmentation of MPOD levels and the observed benefits in BCVA. Several reports seem to mirror these findings, wherein xanthophyll carotenoids notably increased BCVA scores when supplemented for 12 months or longer [
210,
212,
215,
224,
225,
226]. Interestingly, Liu et al. noted that the magnitude of improvement in visual acuity among those with late AMD was substantially reduced, in comparison to those seen in eyes with early or intermediate AMD [
307]. These findings likely underscore the mechanisms of action and criticality of the potential of xanthophyll carotenoids in ameliorating the integrity of the neurosensory retina before permanent loss of macular photoreceptors.
Although these improvements in visual acuity are encouraging, changes in CS function represent more comprehensive outcome measures for detecting subtle alterations in visual capacity, following treatment with carotenoid vitamin therapy. Indeed, CS is a more reliable measure of visual function that captures the essence of spatial visual sensitivity and is often prognosticative of poor visual performance, especially in eyes with maculopathy [
308,
309,
310]. Furthermore, a significant linear association was shown between the positive changes in MPOD and the effects on CS at middle frequency [
307]. In concordance with this meta-analysis, many AMD trials have also reported demonstrable improvements in CS at low and middle spatial frequencies, following significant enhancement in the macular pigment [
53,
115,
117,
204,
205,
224,
225,
226,
228,
231]. Similar to other reports, one study found that, in contrast to the considerable rise in MPOD from baseline to 24 weeks, statistically significant changes in CS were only observed after 48 weeks of supplementation [
115]. Hence, these findings seem to suggest that MPOD status may represent a sine qua non for visual function improvement; for instance, contrast sensitivity would show significant improvement, only after MPOD had been sustained at greater concentrations [
57,
115,
292]. This hypothesis is supported by the functional capacity of the macular pigments, wherein the preferential absorption of short-wavelength (blue) light provides pre-receptoral filtration, in addition to limiting the adverse effects of chromatic aberration [
55,
290,
291,
292,
293,
294,
295,
296,
297,
298,
299]. Greater MPOD levels may also account for the improvements in glare disability [
225,
226] and photostress recovery [
115,
204,
225]. Hence, carotenoid vitamin therapy was shown to significantly ameliorate several measures of visual performance that worsen, with respect to age and in patients with early or late AMD.
Several AMD trials also demonstrated remarkable improvements in objective measurement of macular function, following supplementation with xanthophyll carotenoids for twelve months or more [
211,
218,
220,
223]. Previous studies suggest that the functional integrity of the central retina, particularly the macular region, may be compromised during the early stages of disease progression [
311,
312,
313,
314,
315,
316,
317,
318,
319,
320,
321]. Reports from AMD trials found that carotenoid vitamin therapy offered protection against early functional abnormalities within the central retina (between 0° and 5° eccentricity) [
211,
218,
220,
223]. In fact, two reports indicate that the improvements in central retinal function were positively correlated with MPOD augmentation [
218,
220]. These results may also be attributed, at least in part, to the enhanced neuroprotective capacity, afforded by these dietary antioxidants, to ameliorate pro-oxidative and pro-inflammatory mechanisms in the local tissue, particularly within the neurosensory layers of the macula [
46,
255,
322,
323,
324]. In addition to improving the total antioxidant capacity, xanthophylls may also promote metabolic efficiency of the visual transduction cascade by augmenting mitochondrial dysfunction, a primary source of intracellular free radical formation in aging retina [
42,
46,
47,
48,
255,
298,
324]. It has also been postulated that greater levels of carotenoids may help to promote the maintenance of synaptic network activity by enhancing cell survival and the viability of neurosensory cells [
47,
48,
298]. However, additional studies are needed to further elucidate the potential role of the carotenoids involved with synaptic network activity and cognitive function [
298,
325]. These findings suggest that long-term treatment with carotenoids lutein and zeaxanthin in patients with AMD may promote enhanced retinal function by increasing macular pigment concentrations.
In summary, 21 randomized clinical trials reported on the efficacy of carotenoid vitamin therapy on augmenting MPOD levels (
Table 5), of which 18 studies demonstrated statistically significant improvements [
53,
115,
117,
119,
120,
204,
205,
206,
208,
210,
213,
214,
215,
216,
222,
225,
226,
228,
229,
230,
231]. Similarly, all 15 studies, highlighted in
Table 4, saw demonstrable improvements in the serum concentrations of these xanthophylls, following oral supplementation [
50,
115,
119,
204,
205,
206,
207,
213,
217,
219,
221,
222,
227,
229,
231]. Differential changes in visual performance measures were investigated among 18 studies reporting on visual acuity [
50,
53,
115,
117,
120,
205,
208,
210,
211,
212,
214,
215,
216,
217,
222,
224,
225,
226] and 15 studies reporting on contrast sensitivity function [
53,
115,
117,
204,
205,
209,
210,
211,
216,
224,
225,
226,
228,
229,
231]. Improvements in BCVA were seen in six out of eighteen (6/18) trials [
210,
212,
215,
224,
225,
226]; meanwhile, remarkable benefits in CS were demonstrated in ten out of fifteen (10/15) randomized controlled trials [
53,
115,
117,
204,
205,
224,
225,
226,
228,
231]. Five studies evaluated changes in glare disability [
204,
209,
225,
226,
229], of which four reports indicated significant improvement with carotenoid vitamin therapy [
204,
209,
225,
226]. Similarly, five clinical trials investigated the effect on photostress recovery time in AMD patients [
115,
117,
204,
225,
226], wherein three reports saw changes of statistical significance [
204,
225,
226]. Furthermore, each of the four studies, investigating the objective measures of retinal function through multifocal electroretinogram, showed significant improvements with carotenoid supplementation [
211,
218,
220,
223]. It is noteworthy to point out, as summarized in
Table 5, that various randomized clinical trials demonstrated significant benefits of carotenoid vitamin supplementation in all stages of AMD.
Reports seem to indicate that AMD patients would likely require a minimum of twelve months of using carotenoid vitamin therapy and a higher dose of carotenoids before measurable benefits in visual function would become clinically apparent. The Carotenoids in Age-Related Maculopathy Italian Study (CARMIS) found that the relative risk of three or more letter visual loss was reduced by up to 76% (RR: 0.26; 95% CI: 0.11–0.59) among patients with atrophic AMD, following two years of carotenoid vitamin therapy [
224]. Repeated measures analysis also demonstrated remarkable time effects were seen for the improvements in CS at 6, 12, and 24 months in the active treatment group [
224]. This may explain, at least in part, why some trials with shorter durations reported increases in MPOD but only saw trends of improvement in visual function that did not achieve statistical significance [
117,
120,
214,
216,
217,
222]. However, more recent studies investigating the different ratios of xanthophyll carotenoids in formulation, namely the addition of
meso-zeaxanthin, have largely shown that incorporating all three carotenoids may offer advantages for the management of early AMD [
53,
205,
228,
230].
It is important to note several potential limitations to these studies. In general, the consumption of any single micronutrient-containing vitamin does not appear to afford protection against AMD onset. Although, based on the current evidence, when combined with other antioxidants, dietary carotenoid supplementation with lutein, zeaxanthin, and/or
meso-zeaxanthin does appear to substantially delay the disease progression in established AMD. The studies that looked at the addition of
meso-zeaxanthin to the carotenoid formulation did not explore a separate group with
meso-zeaxanthin alone. So, the exact benefit of including
meso-zeaxanthin is not fully understood, as lutein should theoretically be converted to
meso-zeaxanthin in all individuals that have the RPE65 isomerase. It is unknown (and additional research is needed) if greater amounts of lutein or zeaxanthin may be a sufficient and suitable substitute to
meso-zeaxanthin or if
meso-zeaxanthin is truly needed. It is noteworthy that xanthophyll carotenoids, plus antioxidants, did not exert similar treatment effects on geographic atrophy progression during the five-year follow-up in AREDS2 [
203]. This may be attributed, at least in part, to advanced stages of disease and by poor micronutrient absorption rates, which likely represents a limiting factor and should not be ruled out from clinical trials [
122,
326]. However, one preliminary report indicated that oral zeaxanthin supplementation, as an adjunct to an aggressive triple combination therapy regimen (including bevacizumab, steroid, and photodynamic therapy with verteporfin) for patients with subfoveal choroidal neovascularization, enhanced therapeutic efficiency and decreased the number of treatment cycles required [
327]. Similar benefits were reported in cultured human RPE cells, following hypoxia-induced VEGF secretion, whereby treatment with zeaxanthin was suggested to offer direction protection against the pro-angiogenic factors contributing to neovascular lesions [
328]. Thus, improving carotenoid bioavailability should be among the primary aims for future interventional trials. The bioavailability of carotenoids, following assimilation and transport from dietary matrices, is also strongly influenced by age, gender, and ethnic origin, as well as anthropometric characteristics [
43,
47,
250,
266,
270,
275,
277,
278,
279,
281,
329]. To overcome such limitations, advancements in micronized and nanoemulsion-based micronutrient delivery techniques have demonstrated improved bioavailability and accumulation of xanthophyll carotenoids in the retina, while maintaining overall safety [
53,
122,
270,
330,
331,
332]. Also, the measurement of MPOD longitudinally can provide a measure of “true bioavailability” at the end organ, which is targeted by the carotenoid vitamin therapy.