LRRK2 is a large (2527 amino acids, 286 kDa), multidomain protein, that bears two enzymatic functions: kinase and GTPase, and several protein-protein interaction domains. Numerous genomic LRRK2 variants have been repeatedly confirmed as pathogenic in Parkinson's Disease (PD). Inhibition of LRRK2 was shown to rescue neurite shortening caused by PD mutations in this protein. This entry discusses possible ways of targetting LRRK2 as potential treatment for PD.
- kinase inhibitors
- neurodegenerative diseases
- Parkinson’s disease
- protein–protein interactions
- small GTPases
- LRRK2
1. Overview
Parkinson’s Disease (PD) affects millions of people worldwide with no cure to halt the progress of the disease. Leucine-rich repeat kinase 2 (LRRK2) is the most common genetic cause of PD and, as such, LRRK2 inhibitors are promising therapeutic agents. In the last decade, great progress in the LRRK2 field has been made. As the majority of developed LRRK2 inhibitors are ATP-competitve and shown some safety concerns, here we present alternative ways of targeting LRRK2 (full text [1]). Currently there are three LRRK2-targeting agents in clinical trials, so more developments are predicted in the upcoming years.
2. Genetic causes of Parkinson's Disease
3. LRRK2 targeting agents in clinical trials
4. Other ways of targeting LRRK2
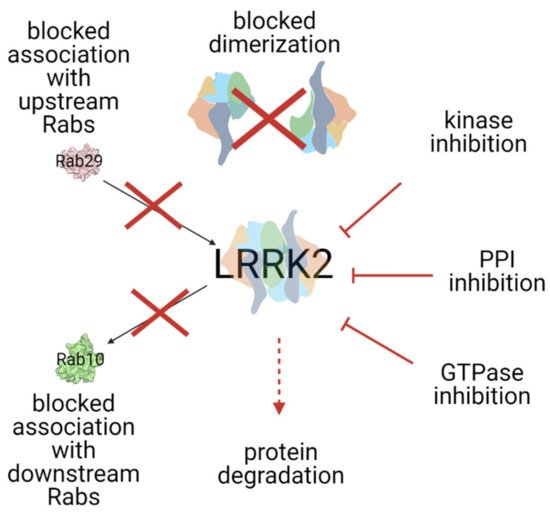
5. Summary and outlook
This entry is adapted from the peer-reviewed paper 10.3390/biom11081101
References
- Dominika Wojewska; Arjan Kortholt; LRRK2 Targeting Strategies as Potential Treatment of Parkinson’s Disease. Biomolecules 2021, 11, 1101, 10.3390/biom11081101.
- E. Ray Dorsey; Todd Sherer; Michael S. Okun; Bastiaan R. Bloem; The Emerging Evidence of the Parkinson Pandemic. Journal of Parkinson's Disease 2018, 8, S3-S8, 10.3233/JPD-181474.
- Eldbjørg Hustad; Jan O. Aasly; Clinical and Imaging Markers of Prodromal Parkinson's Disease. Frontiers in Neurology 2020, 11, 395, 10.3389/fneur.2020.00395.
- Sigurlaug Sveinbjornsdottir; The clinical symptoms of Parkinson's disease. Journal of Neurochemistry 2016, 139, 318-324, 10.1111/jnc.13691.
- Roberta Balestrino; Anthony H.V. Schapira; Parkinson disease. European Journal of Neurology 2019, 27, 27-42, 10.1111/ene.14108.
- Ronald B. Postuma; Dag Aarsland; Paolo Barone; David Burn; Christopher H. Hawkes; Wolfgang Oertel; Tjalf Ziemssen; Identifying prodromal Parkinson's disease: Pre-Motor disorders in Parkinson's disease. Movement Disorders 2012, 27, 617-626, 10.1002/mds.24996.
- Dennis W. Dickson; Parkinson's Disease and Parkinsonism: Neuropathology. Cold Spring Harbor Perspectives in Medicine 2012, 2, a009258-a009258, 10.1101/cshperspect.a009258.
- Edoardo Monfrini; Alessio Di Fonzo; Leucine-Rich Repeat Kinase (LRRK2) Genetics and Parkinson’s Disease. Advances in Neurobiology 2017, 14, 3-30, 10.1007/978-3-319-49969-7_1.
- Dunhui Li; Frank L. Mastaglia; Sue Fletcher; Steve D. Wilton; Progress in the molecular pathogenesis and nucleic acid therapeutics for Parkinson's disease in the precision medicine era. Medicinal Research Reviews 2020, 40, 2650-2681, 10.1002/med.21718.
- Udhaya Kumari; E. K. Tan; LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. The FEBS Journal 2009, 276, 6455-6463, 10.1111/j.1742-4658.2009.07344.x.
- Roberto Di Maio; Eric K. Hoffman; Emily Rocha; Matthew T. Keeney; Laurie H. Sanders; Briana R. De Miranda; Alevtina Zharikov; Amber Van Laar; Antonia F. Stepan; Thomas Lanz; et al. LRRK2 activation in idiopathic Parkinson’s disease. Science Translational Medicine 2018, 10, eaar5429, 10.1126/scitranslmed.aar5429.
- Denali press release 14 January 2020 . Denali Therapeutics. Retrieved 2021-8-6
- Denali press release 6 August 2020 . Denali Therapeutics. Retrieved 2021-8-6
- Denali press release 8 January 2021 . Denali Therapeutics. Retrieved 2021-8-6
- Ahmed Soliman; Fatma Nihan Cankara; Arjan Kortholt; Allosteric inhibition of LRRK2, where are we now. Biochemical Society Transactions 2020, 48, 2185-2194, 10.1042/bst20200424.
- Ying Fan; Francesca Tonelli; Shalini Padmanabhan; Marco A.S. Baptista; Lindsey Riley; Danielle Smith; Connie Marras; Andrew Howden; Dario Alessi; Esther Sammler; et al. Human Peripheral Blood Neutrophil Isolation for Interrogating the Parkinson's Associated LRRK2 Kinase Pathway by Assessing Rab10 Phosphorylation.. Journal of Visualized Experiments 2020, 1, e58956, 10.3791/58956.
- Raja S. Nirujogi; Francesca Tonelli; Matthew Taylor; Pawel Lis; Alexander Zimprich; Esther Sammler; Dario R. Alessi; Development of a multiplexed targeted mass spectrometry assay for LRRK2-phosphorylated Rabs and Ser910/Ser935 biomarker sites. Biochemical Journal 2021, 478, 299-326, 10.1042/bcj20200930.
- Sebastian Virreira Winter; Ozge Karayel; Maximilian Thomas Strauss; Shalini Padmanabhan; Matthew Surface; Kalpana Merchant; Roy N Alcalay; Matthias Mann; Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Molecular Medicine 2021, 13, e13257, 10.15252/emmm.202013257.
