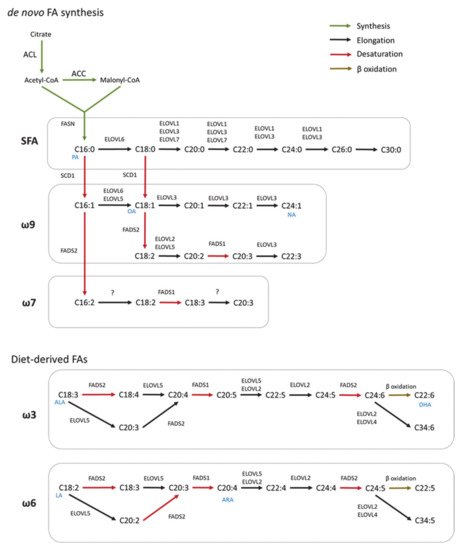
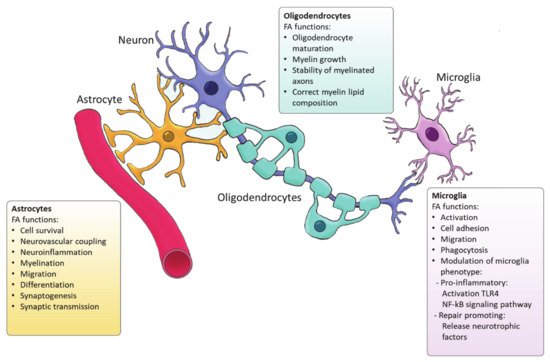
Microglia are the resident immune cells of the CNS and comprise ∼10–20% of all glial cells [
91]. Unlike astrocytes and oligodendrocytes, which are derived from a common lineage of neural progenitor cells, microglia are originated from yolk sac primitive macrophage progenitors that invade the brain at the very early stages of embryonic development. Nowadays, microglia are considered a versatile group of cells [
92,
93]. Microglia provide the first line of innate immunity of the CNS but its functions go beyond scavenging of debris and infectious agents [
92]. Microglia mediate synaptic pruning [
94] and regulate neurogenesis and repair [
95,
96]. Microglia are rich in phosphatidylglycerols and sphingomyelins, containing high levels of specific sphingomyelin which are nearly absent in other glia cells. During the last decade, several studies have demonstrated the importance of FAs in directing microglia function (
Figure 2) [
97]. Microglia can adopt distinctive phenotypes in response to different stimuli with the classically activated inflammatory state and the alternatively activated, inflammation resolving state as the extremes [
7]. The inflammatory phenotype is characterized by the production of pro-inflammatory cytokines and neurotoxic components whereas the alternatively activated phenotype is characterized by the release anti-inflammatory and neurotrophic factors, granting them a repair promoting phenotype [
98,
99,
100]. Yet, the phenotypes found in vivo significantly differ from these two extremes since they display a spatiotemporal spectrum of phenotypes [
98,
101]. Long chain SFAs, for instance palmitic and stearic acids, contribute to a pro-inflammatory phenotype by activating Toll-like receptor 4 (TLR4) and NF-kB signaling pathways [
102,
103,
104,
105]. In contrast, n-3 PUFAs stimulate an anti-inflammatory phenotype [
106]. MUFAs such as oleic acid are described to promote anti-inflammatory processes via activation of the transcription factor peroxisome proliferator-activated receptor [
97]. However, our recent study shows that MUFAs generated by SCD1 can shift microglia and macrophages into an inflammatory phenotype [
107]. Although FAs and their derivatives are crucial for defining microglial function, only few studies describe the involvement of
de novo FA synthesis in directing microglia activity. Despite the lack of studies in microglia, there are extensive studies on FA metabolism in macrophages. Microglia and macrophages share many features and during neuroinflammatory responses macrophages infiltrate the CNS where they, alongside microglia, execute innate effector mechanisms [
108]. Therefore, it can be expected that there are parallels in the regulation of the function of both phagocyte types by FA metabolism. In macrophages, FA synthesis is indispensable for membrane remodeling and the synthesis of inflammatory factors [
109]. Moreover, FASN has shown to be required for inflammatory activation of macrophages. Lack of FASN not only disrupts cell membrane composition by impairing the retention of plasma membrane cholesterol but also alters Rho GTPase trafficking, a process essential for cell adhesion, migration and activation [
110]. Talamonti et al. demonstrated that ELOVL2 deficiency decreases DHA levels in macrophages, affecting their plasticity and promoting a hyperactive inflammatory phenotype [
111]. In addition, microglia can synthesize neuroprotectin PD1 (NPD1) from DHA, a specialized pro-resolving lipid mediator (SPM) [
112]. SPMs are a family of bioactive metabolites generated in response to inflammation by enzymatic of PUFAs [
113]. NPD1 is known to promote phagocytosis and resolve inflammation [
114]. Taken together, while SFA synthesis is suggested to favor inflammatory activation of microglia, PUFAs biosynthesis promotes an anti-inflammatory phenotype, and MUFA synthesis seems to have a dual influence on the microglia phenotype which probably depends on the disease context.