A summary of repellent/deterrent microorganisms involved in mosquito oviposition site selection is detailed in.
- microbiota
- microbiome
- mosquitoes
- behavior
- oviposition
- larval habitat
- life history traits
- nutrition
- development
- survival
1. Introduction
Amongst arthropods, mosquitoes (Diptera: Culicidae) form a highly diversified family with more than 3601 different species divided into two different sub-families: Anophelinae (482 species) and Culicinae (3119 species) [1]. Mosquitoes are the major disease vectors worldwide with some species being able to transmit pathogens of public and veterinary importance. For example, Aedes mosquitoes transmit arboviruses including dengue, chikungunya, and yellow fever viruses while Anopheles are the vectors of Plasmodium spp. parasites responsible for malaria [2]. Several physiological, ecological, and environmental factors impact the probability of mosquitoes to transmit pathogens in the field such as (i) vector density and biting rates, (ii) pathogen survival, (iii) host-vector contact as well as (iv) insect vector competence. The latter is defined as the ability of pathogens to efficiently colonize the vector, to replicate and get transmitted under controlled conditions [3]. Therefore, limiting the density of vector populations below the transmission threshold (i.e., the critical level of vector density above which the introduction of a few infectious individuals into a community of susceptible individuals will give rise to an outbreak) is a keystone action that can be performed in order to limit the expansion of mosquito-borne diseases. To that end, methods mainly based on the use of chemical insecticides have been applied to control mosquitoes. As an example, the Center for Disease Control and Prevention (Atlanta, GA, USA) recommends their use inside housing in order to limit malaria transmission. Such a strategy has led to a 21% decrease of malaria cases over the world between 2012 and 2015 [2]. Despite their proven efficiency, chemical insecticides often (i) lack specificity and impact on untargeted species, (ii) led to the selection of mosquito resistant populations as previously evidenced for dichlorodiphenyltrichloroethane (DDT) and pyrethroids, (iii) led to health issues, in particular when they are used indoor [4][5][6]. To overcome these undesirable effects, alternative strategies have gradually been developed. Among them, insect-chemoattractant/repellent compounds as well as organic insecticides, most often originating from microorganisms, have been applied in the field [7][8][9][10][11].
Mosquitoes are holometabolous insects meaning that they will proceed to a complete metamorphosis. After the egg has hatched in aquatic environment, individuals will follow a post-embryonic development starting with a larval stage and a pupal stage to finally emerge as an imago. Each stage but imago colonizes aquatic habitats. Larvae use different feeding strategies such as filtering, suspension feeding, grazing, interfacial feeding, or predation, to acquire organic matters within their aquatic habitats [12]. They developed into four different instars that are separated by exuviations and metamorphose into pupae before emerging as an adult at the interface between air and water. After being mated by males, females of anautogenous species (most species such as Aedes albopictus ) will bite a vertebrate host in order to acquire essential amino acids required for egg maturation [13]. Conversely, autogenous species ( Malaya spp., Toxorynchites spp. , and Topomyia spp.) can lay eggs without ingesting any blood meal. Recognition and selection of breeding sites by gravid females is a key step in mosquito life cycles. Since a single mosquito female lays multiple clutches during its whole life and since each clutch is ranging from tens to hundreds of eggs without no parental care, it is of primary importance to manage larval habitats. For instance, An. gambiae females can delay egg laying up to 50 days in absence of suitable breeding sites [14]. This drastically impacts the fitness of individuals by reducing egg hatching and larval development rates. Even if all mosquitoes are selecting aquatic habitats, each species search for and select certain characteristics of these habitats (e.g., in term of salinity, sunlight exposition, stream flow, type of predators…) [13][15]. As an example, the mosquito species Aedes taeniorhynchus and Anopheles crucians tend to prefer domestic habitats and lay eggs in artificial containers while other species such as Culiseta melanura prefer sylvatic sites and freshwater swamps [16].
Egg laying site selection is a keystone behavior determining the fate of the female progeny and, thus, is expected to be under strong selective pressures. Such localization and selection of water habitats by gravid females involve olfactory, visual, gustatory, and tactile signals [17]. Mosquitoes detect olfactory signals with their antennae, maxillary palps, and proboscis [18]. Tarsal segments of the legs, the labellum and labrum of the mouthparts, and the cibarium, an internal organ, are rather important for tasting and sensing the breeding site [19]. These organs contain multiporous sensory hairs called sensilla that house olfactory sensory neurons expressing chemosensory receptors that are detecting specific compounds. Phenotypic responses of gravid females to environmental signals might vary. Some signals can be classified as (i) “attractant” if they elicit insect-oriented movement toward the source, (ii) “repellent” if they induce insect-oriented movement away from the source, (iii) “stimulant” if they elicit oviposition, and (iv) “deterrent” if they prevent oviposition ( Figure 1 ; [20]). Those water habitats are colonized by a wide variety of prokaryotic and eukaryotic microorganisms. Due to their ability to synthesize compounds with organoleptic properties, they have been shown to influence the mosquito oviposition site selection.
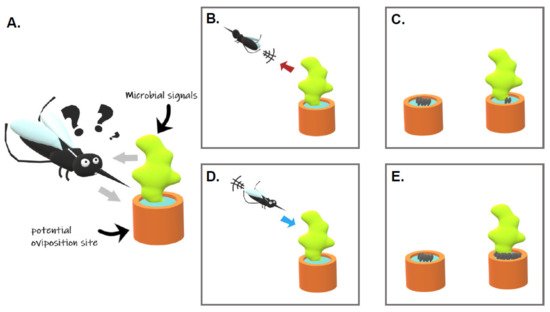
2. Influence of Microorganisms on the Mosquito Oviposition Site Selection
Mosquitoes water habitats are often rich organic matter acquired from soil, vegetation, animal cadavers, and dejections [12]. Such microenvironments promote the growth of a wide variety of microorganisms, which have been shown to be key drivers for communities assembly of mosquitoes microbiota and determine major adult traits [21][22][23][24][25][26]. In this section, we review how microorganisms’ cues either attract/stimulate or repel/deter gravid females. We sum up the knowledge about the characteristics of these microbial kairomones (i.e., semiochemical compounds that are produced by microorganisms and recognized by mosquitoes) and discuss how variations in microbial densities might elicit drastically contrasted behavioral responses in mosquitoes.
Even if plant infusion and their associated microorganisms were shown to be good elicitors of mosquitoes’ oviposition, natural breeding sites often contained a more variable diversity and abundance of microorganisms. Therefore, to mimic natural conditions, other authors tested the effect of water from natural oviposition sites without a priori on the nature of water [27]. They showed that fresh soils or water collected in known oviposition sites of the malaria vector An. gambiae received, respectively, 3.9 and 2.6 times more eggs than sterile distilled water when the choice was offered to gravid females in dual choice experiments. To ensure that only olfaction rather than touching and tasting could be involved in the recognition, the authors used an experimental system preventing female mosquitoes from touching the substrate. Similar results were obtained using sterilized substrate (autoclaved soil of filtered water) instead of sterile water. Isolated bacteria (including unclassified Firmicutes, Aeromonas, Pasteurella, Pseudomonas, Vibrio, Acinetobacter , and Enterobacteriaceae species) from soil collected beneath oviposition sites and larval habitats restored the attractiveness/stimulant properties of sterile soils but not filtered distilled water. These results suggest that the dilution of microorganisms or volatile organic compounds (VOC) into water might decrease the capacity of mosquitoes to use kairomones as an information source. Volatiles from bacteria isolated in this experiment were then analyzed [28]. It appeared that the bacteria correlated with a positive oviposition response clustered into different groups. The authors suggest that different molecules produced by those bacteria and recognized by the mosquito might differ across bacterial isolates. When combined with previous results obtained from mosquito antennae electro-physiological response studies toward volatiles, a list of potential attractive compounds was updated and restricted to aliphatic alcohols (2-Methyl-3-decanol, methyl-1-butanol), aromatic alcohols (2-phenylethanol, phenylmethanol), indole, pyrazines (alkyl-pyrazines), and carboxylic acids (3-methylbutanoic acid). More recently, lake water supplemented with six days-old soil infusions from breeding sites was shown to efficiently attract gravid Anopheles gambiae s.l . females [29]. However, this attractiveness disappeared after autoclaving the mixture. The authors characterized cedrol, a sesquiterpene alcohol, as a major attractant present in the infusion and showed that natural habitats in which cedrol was identified were more likely to be colonized by Anopheles mosquitoes [30]. Finally, they identified two endophytic fungi (a species of the Fusarium fujikuroi complex and F. falciforme ) from rhizomes in soils beneath Anopheles oviposition sites, able to produce cedrol and some of its analogues [31]. This set of results represents major advances in the identification of the molecules or blend that attract female mosquitoes. However, the list is certainly far from exhaustive. Indeed, field surveys often reported that many presumably suitable breeding sites for Anopheles mosquitoes remained uncolonized [32][33][34]. Those observations suggest that important factors influencing breeding site selection might be missing to predict the attractiveness and potential suitability of those habitats.
The microsporidian parasite Edhazardia aedis is an intracellular obligate parasite that specifically infects the mosquito Ae. aegypti [35]. This parasite strongly affects the survival and reproductive success of the mosquito [36]. Its life cycle is complex since the microsporidian spores can be both vertically and horizontally transmitted with a high transmission success [37]. Due to its high transmission rate and maintenance in mosquito populations, the parasite was proposed as a promising candidate for mosquito biological control [38][39]. However, the ability of uninfected Ae. aegypti females to avoid egg deposition when oviposition sites are colonized by infected conspecific larvae questions its use [40]. Indeed, dual choice experiments demonstrated that uninfected females laid a higher proportion of eggs (60.8 ± 2.1%) in cups containing uninfected larvae. The potential semiochemicals involved in attractiveness differentiation were not identified to date. Such a strategy might be an evolutionary response of the mosquito toward the fitness cost of the parasite in natural populations. However, the oviposition deterrence is not complete, which also suggests that in the field, a part of the population will get infected, enabling the parasite to complete its lifecycle and spread among individuals when a part of the population remains uninfected. The trematode Plagiorchis elegans is another parasite of Ae. aegypti . The presence of this parasite in the water, or in a snail host living in aquatic habitats, does not seem to affect the oviposition behavior of gravid females [41]. However, as previously described for E. aedis dual choice experiments showed that breeding sites containing infected larvae were repellent/deterrent toward gravid females and accumulated fewer eggs than sites containing uninfected larvae or solely water [41][42]. This repellent/deterrent effect was still observed when water was treated with antibiotics or boiled, suggesting that (i) presence of the parasite was not mandatory and (ii) that thermostable non-volatile compounds have been produced by infected larvae or by the parasite to mediate breeding site recognition by mosquitoes. In addition, the repellent/deterrent effect was increased when water was filter sterilized, with 10 times more eggs in containers with uninfected larvae. This difference was attributed to bacteria colonizing the containers, such as Flavobacteria sp., that attract mosquitoes, thus, mitigated the repellency of the parasite. Contrarily to this previous experiment where water was regularly changed, a recent study conducted with water that was not changed for 14 days and potentially accumulated bacteria, failed to observe the repellent/deterrent effect of P. elegans infected mosquitoes [43]. This confirms that, due to presence of bacteria in water containers, repellency/deterrence of the parasite might often be mitigated and has rarely been observed in the field. Since Ae. aegypti lay eggs in standing water, it may be possible that the potential repellency/deterrent effect of P. elegans would not be efficient in the field. Bacillus thuringiensis var. israelensis (Bti) is a dipteran pathogen that has been broadly used in biological control against Aedes, Culex, or Anopheles mosquitoes [44]. Depending on the species, female mosquitoes do not respond similarly to the presence of Bti in water habitats. Indeed, Culex quinquefasciatus tend to lay less eggs in Bti-infected water containers compared to sterile water [45]. In addition, the number of eggs laid as well as the size of egg rafts negatively correlated with the concentration of Bti. On the opposite, no influence of Bti was observed toward An. arabiensis female behavior [46] and from no effect to a slight attractive/stimulant effect was even reported for Ae. albopictus [47][48]. Those differences might be explained by the fact that Culex mosquitoes drink water before laying eggs and might recognize solubilized compounds with their phagoreceptors as previously discussed [40]. However, those conclusions should be taken cautiously because different dose of Bti were used in those experiments and mosquito species effects might be confounded with dose effects, which could have also led to differences in gravid female responses. A summary of repellent/deterrent microorganisms involved in mosquito oviposition site selection is detailed in Table 1 .
| Microorganisms | Species | Condition/Concentration | Mosquito Species | Semiochemicals | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aedes aegypti | Aedes albopictus | Anopheles gambiae | An. arabiensis | Culex quinquefasciatus | |||||
| Bacteria | Bacillus thuringiensis | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | |
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Bacillus thuringiensis var. israelensis | 0.5–2 mg/L (for Cx. quinquefasciatus), 8 mg/L (for Ae. albopictus), 2–6 mg/L (for An. arabiensis) | − | no response or attractivity/stimulation | − | no response | repellency/deterrence | [50,51,52,53] | ||
| Brevundimonas vesicularis | 106 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | no response | − | − | − | ||||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Citrobacter freundii | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| Comamonas spp | [4.2 × 107; 8.1 × 107] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Enterobacter asburiae | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Enterobacter cancerogenus | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Enterobacter gergoviae | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Enterobacter ludwigii | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | no response | attractivity/stimulation | − | − | − | ||||
| Exiguobacterium spp | [5.2 × 107; 5.3 × 107] CFU/mL | − | − | attractivity/stimulation | - | - | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Lactococcus lactis | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| Micrococcus. spp | [7.7 × 106; 1.8 × 107] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Proteus spp | [6.9 × 107; 3.2 × 108] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Pseudomonas fulva | 107 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Pseudomonas plecoglossicida | 106 CFU/mL | no response | repellency/deterrence | − | − | − | [49] | ||
| 107 CFU/mL | no response | attractivity/stimulation | − | − | − | ||||
| Rhizobium huautlense | 108 CFU/mL | repellency/deterrence | no response | − | − | − | [49] | ||
| Shigella dysenteriae | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Vibrio metschnikovii | [2 × 108; 4 × 108] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Fungi | Fusarium fujikuroi complex | − | − | attractivity/stimulation | − | − | Cedrol | [43] | |
| Fusarium falciforme | − | − | attractivity/stimulation | − | − | ||||
| Smittium morbosum | infected larvae | repellency/deterrence | − | − | − | − | [48] | ||
| Candidatus near pseudoglaebosa | infected larvae | attractivity/stimulation | − | − | − | − | |||
| Edhazardia aedis | repellency/deterrence | − | − | − | − | [54] | |||
| Protist | Ascogregarina taiwanensis | infected larvae (12–97 trophozoites) | attractivity/stimulation | − | − | − | − | [48] | |
| Trematode | Plagiorchis elegans | infected larvae | repellency/deterrence | − | − | − | − | [55,56] | |
Those results point out that variation in microbial communities’ composition and density shape mosquito oviposition behavior by impacting the diversity and concentration of volatile compounds to either influence the behavior of gravid females. Therefore, identifying the volatile molecules and their dynamics in natural oviposition sites could be key to improve vector control strategies.
3. Influence of Microorganisms Colonizing Water Habitats on Mosquitoes’ Premature Life History Traits
If the importance of microorganisms in the performance–preference coupling has been poorly addressed, several studies previously demonstrated that microbes colonizing water habitats influence the life history traits of mosquitoes with, even, drastic consequences on adult traits (see an example here [23]). In this section, we will more specifically comment the impact of microorganisms on larval nutrition, mosquito development (including egg hatching and post-embryonic development), and immature (eggs and larvae) survival.
If nutrient acquired from digested microbes can increase larval growth non-digested microbes have also been shown to influence the mosquitoes’ development.
All in all, the current literature shows that microorganisms play an important role in the oxygen signals determining egg hatching but other microorganism mediated stimuli should be further investigated.
Those results highlighted the major influence of microorganisms on the signal leading to larval development with consequences on the adult traits. However, most of those effects are not specific enough and further studies are necessary to determine to which extent microbial composition and density modulate the development of larvae.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9081589
References
- Wilkerson, R.C.; Linton, Y.-M.; Fonseca, D.M.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini That Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS ONE 2015, 10, e0133602.
- WHO. World Malaria Report 2016. Available online: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (accessed on 28 December 2020).
- Shaw, W.R.; Catteruccia, F. Vector Biology Meets Disease Control: Using Basic Research to Fight Vector-Borne Diseases. Nat. Microbiol. 2019, 4, 20–34.
- Ranson, H.; Abdallah, H.; Badolo, A.; Guelbeogo, W.M.; Kerah-Hinzoumbé, C.; Yangalbé-Kalnoné, E.; Sagnon, N.; Simard, F.; Coetzee, M. Insecticide Resistance in Anopheles Gambiae: Data from the First Year of a Multi-Country Study Highlight the Extent of the Problem. Malar. J. 2009, 8, 299.
- Benelli, G.; Mehlhorn, H. Declining Malaria, Rising of Dengue and Zika Virus: Insights for Mosquito Vector Control. Parasitol. Res. 2016, 115, 1747–1754.
- Demok, S.; Endersby-Harshman, N.; Vinit, R.; Timinao, L.; Robinson, L.J.; Susapu, M.; Makita, L.; Laman, M.; Hoffmann, A.; Karl, S. Insecticide Resistance Status of Aedes Aegypti and Aedes Albopictus Mosquitoes in Papua New Guinea. Parasites Vectors 2019, 12, 333.
- Logan, J.G.; Birkett, M.A. Semiochemicals for Biting Fly Control: Their Identification and Exploitation. Pest Manag. Sci. 2007, 63, 647–657.
- Bhattacharya, P.R. Microbial Control of Mosquitoes with Special Emphasis on Bacterial Control. Indian J. Malariol. 1998, 35, 206–224.
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites Vectors 2013, 6, 1.
- Dickens, J.C.; Bohbot, J.D. Mini Review: Mode of Action of Mosquito Repellents. Pestic. Biochem. Physiol. 2013, 106, 149–155.
- Guégan, M.; Zouache, K.; Démichel, C.; Minard, G.; Tran Van, V.; Potier, P.; Mavingui, P.; Valiente Moro, C. The Mosquito Holobiont: Fresh Insight into Mosquito-Microbiota Interactions. Microbiome 2018, 6, 49.
- Capinera, J.L. (Ed.) Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-0-306-48380-6.
- Clements, A.N. The Biology of Mosquitoes: Development, Nutrition and Reproduction; Chapman & Hall: London, UK, 1992; ISBN 978-0-412-40180-0.
- Touré, D.S.; Ouattara, A.F.; Kra, K.D.; Kwadjo, K.E.; Koné, M.; Doumbia, M.; Doannio, J.M.C. Impact of egg laying delay on reproduction, gorging habit and mortality in gravid females Anopheles gambiae (Diptera Culicidae). Bull. Soc. Pathol. Exot. 2017, 110, 318–325.
- Clements, A.N. The Biology of Mosquitoes: Sensory Reception and Behaviour; CABI: Wallingford, UK, 1999; ISBN 978-0-85199-313-3.
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65.
- Bentley, M.D.; Day, J.F. Chemical Ecology and Behavioral Aspects of Mosquito Oviposition. Annu. Rev. Entomol. 1989, 34, 401–421.
- McMeniman, C.J. Chapter 11—Disruption of Mosquito Olfaction. In Genetic Control of Malaria and Dengue; Adelman, Z.N., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 227–252. ISBN 978-0-12-800246-9.
- Baik, L.S.; Carlson, J.R. The Mosquito Taste System and Disease Control. Proc. Natl. Acad. Sci. USA 2020, 117, 32848–32856.
- Dethier, V.G.; Browne, B.L.; Smith, C.N. The Designation of Chemicals in Terms of the Responses They Elicit from Insects. J. Econ. Entomol. 1960, 53, 134–136.
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes Rely on Their Gut Microbiota for Development. Mol. Ecol. 2014, 23, 2727–2739.
- Dada, N.; Jumas-Bilak, E.; Manguin, S.; Seidu, R.; Stenström, T.-A.; Overgaard, H.J. Comparative Assessment of the Bacterial Communities Associated with Aedes Aegypti Larvae and Water from Domestic Water Storage Containers. Parasites Vectors 2014, 7, 391.
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover Effects of Larval Exposure to Different Environmental Bacteria Drive Adult Trait Variation in a Mosquito Vector. Sci. Adv. 2017, 3, e1700585.
- Minard, G.; Tran, F.-H.; Van, V.T.; Fournier, C.; Potier, P.; Roiz, D.; Mavingui, P.; Moro, C.V. Shared Larval Rearing Environment, Sex, Female Size and Genetic Diversity Shape Ae. Albopictus Bacterial Microbiota. PLoS ONE 2018, 13, e0194521.
- Alfano, N.; Tagliapietra, V.; Rosso, F.; Manica, M.; Arnoldi, D.; Pindo, M.; Rizzoli, A. Changes in Microbiota Across Developmental Stages of Aedes Koreicus, an Invasive Mosquito Vector in Europe: Indications for Microbiota-Based Control Strategies. Front. Microbiol. 2019, 10, 2832.
- Nilsson, L.K.J.; de Oliveira, M.R.; Marinotti, O.; Rocha, E.M.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Characterization of Bacterial Communities in Breeding Waters of Anopheles Darlingi in Manaus in the Amazon Basin Malaria-Endemic Area. Microb. Ecol. 2019, 78, 781–791.
- Sumba, L.A.; Guda, T.O.; Deng, A.L.; Hassanali, A.; Beier, J.C.; Knols, B.G.J. Mediation of Oviposition Site Selection in the African Malaria Mosquito Anopheles Gambiae (Diptera: Culicidae) by Semiochemicals of Microbial Origin. Int. J. Trop. Insect Sci. 2004, 24, 260–265.
- Lindh, J.M.; Kännaste, A.; Knols, B.G.J.; Faye, I.; Borg-Karlson, A.-K. Oviposition Responses of Anopheles Gambiae s.s. (Diptera: Culicidae) and Identification of Volatiles from Bacteria-Containing Solutions. J. Med. Entomol. 2008, 45, 1039–1049.
- Herrera-Varela, M.; Lindh, J.; Lindsay, S.W.; Fillinger, U. Habitat Discrimination by Gravid Anopheles Gambiae Sensu Lato—A Push-Pull System. Malar. J. 2014, 13, 1–15.
- Lindh, J.M.; Okal, M.N.; Herrera-Varela, M.; Borg-Karlson, A.-K.; Torto, B.; Lindsay, S.W.; Fillinger, U. Discovery of an Oviposition Attractant for Gravid Malaria Vectors of the Anopheles Gambiae Species Complex. Malar. J. 2015, 14, 119.
- Eneh, L.K.; Saijo, H.; Borg-Karlson, A.-K.; Lindh, J.M.; Rajarao, G.K. Cedrol, a Malaria Mosquito Oviposition Attractant Is Produced by Fungi Isolated from Rhizomes of the Grass Cyperus Rotundus. Malar. J. 2016, 15, 478.
- Fillinger, U.; Sombroek, H.; Majambere, S.; van Loon, E.; Takken, W.; Lindsay, S.W. Identifying the Most Productive Breeding Sites for Malaria Mosquitoes in The Gambia. Malar. J. 2009, 8, 62.
- Ndenga, B.A.; Simbauni, J.A.; Mbugi, J.P.; Githeko, A.K.; Fillinger, U. Productivity of Malaria Vectors from Different Habitat Types in the Western Kenya Highlands. PLoS ONE 2011, 6, e19473.
- Gouagna, L.C.; Rakotondranary, M.; Boyer, S.; Lempérière, G.; Dehecq, J.-S.; Fontenille, D. Abiotic and Biotic Factors Associated with the Presence of Anopheles Arabiensis Immatures and Their Abundance in Naturally Occurring and Man-Made Aquatic Habitats. Parasites Vectors 2012, 5, 96.
- Becnel, J.J.; Johnson, M.A. Mosquito Host Range and Specificity of Edhazardia Aedis (Microspora: Culicosporidae). J. Am. Mosq. Control Assoc. 1993, 9, 269–274.
- Becnel, J.J.; Garcia, J.J.; Johnson, M.A. Edhazardia Aedis (Microspora: Culicosporidae) Effects on the Reproductive Capacity of Aedes Aegypti (Diptera: Culicidae). J. Med. Entomol. 1995, 32, 549–553.
- Agnew, P.; Koella, J. Life History Interactions with Environmental Conditions in a Host–Parasite Relationship and the Parasite’s Mode of Transmission. Evol. Ecol. 1999, 13, 67–91.
- Becnel, J.J.; Johnson, M.A. Impact of Edhazardia Aedis (Microsporidia: Culicosporidae) on a Seminatural Population of Aedes Aegypti (Diptera: Culicidae). Biol. Control 2000, 18, 39–48.
- Grigsby, A.; Kelly, B.J.; Sanscrainte, N.D.; Becnel, J.J.; Short, S.M. Propagation of the Microsporidian Parasite Edhazardia Aedis in Aedes Aegypti Mosquitoes. J. Vis. Exp. 2020, e61574.
- Zettel Nalen, C.M.; Allan, S.A.; Becnel, J.J.; Kaufman, P.E. Oviposition Substrate Selection by Florida Mosquitoes in Response to Pathogen-Infected Conspecific Larvae. J. Vector Ecol. 2013, 38, 182–187.
- Lowenberger, C.A.; Rau, M.E. Selective Oviposition by Aedes Aegypti (Diptera: Culicidae) in Response to a Larval Parasite, Plagiorchis Elegans (Trematoda: Plagiorchiidae). Environ. Entomol. 1994, 23, 1269–1276.
- Zahiri, N.; Rau, M.E. Oviposition Attraction and Repellency of Aedes Aegypti (Diptera: Culicidae) to Waters from Conspecific Larvae Subjected to Crowding, Confinement, Starvation, or Infection. J. Med Entomol. 1998, 35, 782–787.
- Schwab, A.E.; Lewis, D.J.; Rau, M.E. The Impact of Selective Oviposition and Infection with Plagiorchis Elegans on Aedes Aegypti Pre-Imago Population Dynamics at Optimal Food Availability. J. Med. Entomol. 2003, 40, 830–840.
- Ben-Dov, E. Bacillus Thuringiensis Subsp. Israelensis and Its Dipteran-Specific Toxins. Toxins 2014, 6, 1222–1243.
- Zahiri, N.S.; Mulla, M.S. Ovipositional and Ovicidal Effects of the Microbial Agent Bacillus thuringiensis israelensis on Culex quinquefasciatus Say (Diptera: Culicidae). J. Vector Ecol. 2006, 31, 29–34.
- Futami, K.; Kongere, J.O.; Mwania, M.S.; Lutiali, P.A.; Njenga, S.M.; Minakawa, N. Effects of Bacillus thuringiensis Israelensis on Anopheles arabiensis. J. Am. Mosq. Control Assoc. 2011, 27, 81–83.
- Stoops, C.A. Influence of Bacillus Thuringiensis Var. Israelensis on Oviposition of Aedes Albopictus (Skuse). J. Vector Ecol. 2005, 30, 41–44.
- Wasi Ahmad, N.; Lee, H.; Wan, R.; Lian, A.; Chee Dhang, C.; Azahari, A.; Sadiyah, I. Oviposition Behaviour of Aedes Albopictus in Temephos and Bacillus Thuringiensis Israelensis-Treated Ovitraps. Dengue Bull. 2009, 33, 209–217.
