Fluid pressure is elevated in the interstitial tissue of tumors [
14]. For example, interstitial fluid pressure values as high as 33 mmHg have been recorded in some sarcomas [
15]. Several studies indicate that high interstitial fluid pressure in the tumor is correlated with poor prognosis [
16]. Most drugs used for systemic treatment of patients with cancer—high-molecular-weight compounds in particular—are transported from the circulatory system through the interstitial space by convection, that is, they are carried by streaming of a flowing fluid [
17]. Thus, increased interstitial fluid pressure within the tumor node causes less uptake of drugs into the tumor. Moreover, in PM, interstitial fluid pressure profiles vary between different tumor zones [
13], suggesting that exposition of tumor cells to chemotherapeutic drugs might vary within the metastasis.
During tumor progression the extracellular matrix undergoes so-called mesothelial–mesenchymal transition (MMT) [
18]. MMT is characterized by the progressive replacement of normal peritoneal mesothelial cells tissue by fibroblasts, a major cellular component of scar tissue [
19]. During MMT, peritoneal mesothelial cells lose their epithelial-like characteristics, including cell–cell junctions, tight junctions, adherence junctions and desmosomes, lose their apicobasal polarity and progressively acquire a mesenchymal phenotype, characterized by actin reorganization, stress fiber formation, migration, and invasion [
20]. These dramatic changes in tissue architecture, formerly named “desmoplasia”, are most pronounced at the tumor invasion front. Due to tissue remodeling and increased interstitial fluid pressure (IFP), drug uptake is less effective into hard, fibrotic tissue than into normal tissue. This results in increased resistance to chemotherapy, which is largely independent of the mode of action of the drug applied.
2. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
PIPAC is a minimally invasive approach relying on physical principles for improving intraperitoneal drug delivery, including: (1) optimizing the homogeneity of drug distribution by applying an aerosol rather than a liquid solution; (2) applying increased intraperitoneal hydrostatic pressure to counteract elevated intratumoral interstitial fluid pressure; (3) limiting blood outflow during drug application; (4) steering environmental parameters (temperature, pH, electrostatic charge etc.) in the peritoneal cavity for best tissue target effect. In addition, PIPAC allows repeated application and objective assessment of tumor response by comparing biopsies between chemotherapy cycles.
Staging laparoscopy has developed to be a standard of care in ovarian cancer [
48] and in gastric cancer [
49]. During such procedures, carbon dioxide (CO
2) pneumoperitoneum is applied in order to create a working space. This working space allows for the safe placement of access ports through the abdominal wall [
50], visualization of organs [
48,
51] and completion of complex interventions [
52,
53]. Twenty years ago, we developed a first-generation device suitable for minimally invasive surgery procedures that allowed microdroplets of a therapeutic substance to be distributed into the pneumoperitoneum (CO
2), creating a "therapeutic pneumoperitoneum" [
54].
Over the years, in addition to the aerosolization of a drug, we found it useful to add two further components, namely steering the operating environment (pressure, temperature, electrostatic charges, etc.) and objective assessment of tumor response (by comparing biopsies between repeated applications) [
55,
56]. All three components are now part of PIPAC [
57]. Starting with the first preclinical experiments through technology development, first in-human use [
58], Phase 1 [
59,
60,
61] and Phase 2 [
62,
63,
64,
65,
66,
67] trials, PIPAC is currently being evaluated in randomized controlled trials [
68,
69,
70,
71,
72] for palliative therapy of PM [
73].
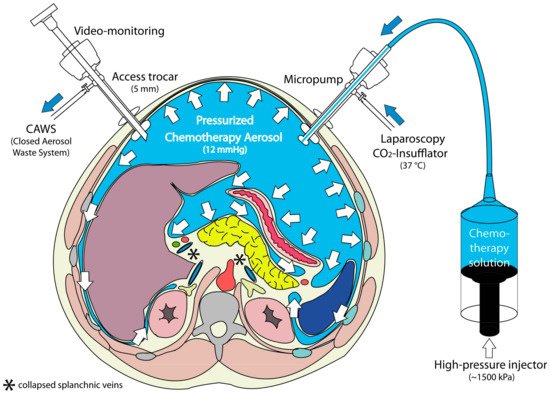
PIPAC (Figure 3) is applied through laparoscopic access using two balloon trocars in an operating room equipped with laminar air flow. In a first step, a normothermic capnoperitoneum is established with a pressure of 12 mmHg. A cytotoxic solution (about 10–20% of a normal systemic dose) is nebulized with a micropump into the abdominal cavity and maintained for 30 min. The aerosol is then removed through a closed suction system.
Figure 3. Principle of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). During a staging laparoscopy, an aerosol cytostatic agent is applied in the abdominal space using a nebulizer. The application as an aerosol allows the relatively even distribution of the substance. Increased pressure (12 mmHg) ensures deeper penetration into the tissue. Reproduced with permission from [
58].
In contrast to inhalers commonly used in pulmonary medicine, no propellant gas is needed. During PIPAC, a liquid solution is aerosolized into the gaseous (CO2) environment using a specific nozzle (Capnopen®, Capnomed, Zimmern, Germany). Energy is provided by applying an upstream mechanical force gradient provided by an industry-standard angioinjector (e.g., Accutron HP®, MedTron, Saarbrücken, Germany).
Theoretical considerations suggest that the therapeutic CO
2-pneumoperitoneum (“capnoperitoneum”) should be capable of carrying microdroplets of active substances to all exposed peritoneal surfaces. These considerations were confirmed by several preclinical experiments, showing that the active principle is distributed, reaching all exposed [
28,
74] and even partially hidden surfaces [
75].
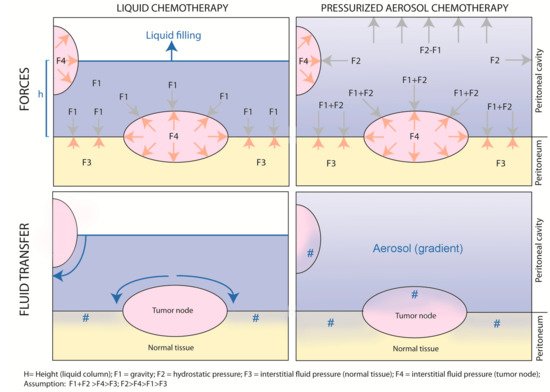
Figure 4 provides a graphical abstract of the differences between non-pressurized, liquid intraperitoneal chemotherapy vs. pressurized, aerosolized intraperitoneal chemotherapy.
Figure 4. “Intraperitoneal chemotherapy with normobaric liquids vs. pressurized aerosols. Graphical abstract of the physical differences between non-pressurized, liquid intraperitoneal chemotherapy vs. pressurized, aerosolized intraperitoneal chemotherapy.” #: drug penetration into the tissue.
As shown in the upper left panel, during liquid intraperitoneal chemotherapy, gravity forces applied to the tumor node depend on the height of the water column (h); the exposition of the tumor node depends on the filling volume. This reflects the situation of open HIPEC. In contrast, during PIPAC (upper right panel), tumor nodes are exposed to unidirectional gravitational and multidirectional hydrostatic forces caused by the pressurized environment. Although an aerosol gradient exists, all exposed peritoneal surfaces are reached by the drug. The net fluid transfer, as illustrated in the lower panels, results from the balance between gravitational and hydrostatic forces on the one hand and tumor node resistance resulting from increased interstitial fluid pressure on the other hand. As shown in the lower left panel, since resistance in normal tissue is lower than in tumor nodes, liquid chemotherapy will follow the path of least resistance and preferentially enter the normal tissue. In contrast (right lower panel), aerosolized chemotherapy will penetrate both the normal and the tumoral tissue, penetrating the normal deeper than the tumoral tissue.
3. Preclinical Evidence of PIPAC
Several studies have evaluated drug uptake into the target tissue after PIPAC, and some of them have compared PIPAC with other drug delivery techniques such as HIPEC and/or laparoscopic HIPEC. Therapeutic substances investigated include methylene blue [
28,
76], DNA [
77], RNA [
29,
78], cisplatin [
79,
80,
81,
82], doxorubicin [
75,
83,
84,
85], oxaliplatin [
82,
86,
87,
88] and liposomal doxorubicin [
89,
90].
3.1. Effect of Hydrostatic Pressure
Increasing intraabdominal hydrostatic pressure during PIPAC significantly enhanced tumor cell toxicity of oxaliplatin in both wild-type and chemotherapy-resistant cells in vitro. A maximum cytotoxicity was observed at 15 mmHg. Pressures >15 mmHg did not show additional cytotoxic effect on cells [
88]. Similarly, increasing PIPAC pressure over 15 mmHg did not increase the depth of doxorubicin tissue penetration in an ex vivo model [
84].
3.2. Homogeneity of Distribution
Delivery of methylene blue into a large animal model unraveled a more homogeneous and more intensive vital staining of the abdominal cavity after aerosolization than after lavage [
28]. Ex vivo experiments on surgical specimens of human peritoneal metastases evidenced a homogeneous distribution of small DNA fragments (Dbait) onto the peritoneum as compared to conventional peritoneal lavage [
77]. In a rodent model, bioluminescence analysis showed a superior distribution of RNA complexes after intraperitoneal nebulization as compared to intraperitoneal injection of liquid: bowel exposition to the therapeutic substance was superior after PIPAC (median: 50%) vs. intraperitoneal injection (median: 10%) [
29]. In a postmortem swine model, doxorubicin administered as PIPAC reached all areas within the peritoneum, with the highest depth of penetration being measured in the area located in front of the aerosolizer [
85]. In two studies, the aerosol reached covered peritoneal areas [
75,
84]. In a living swine model, homogeneity of drug repartition (as defined by the comparison of the oxaliplatin concentration in the parietal vs. visceral peritoneum) was better after PIPAC (ratio: 11.5) than after HIPEC (ratio: 17.6) [
30].
3.3. Tissue Concentration
All pharmacological data published so far show a superior therapeutic ratio (tissue concentration/dose applied) of PIPAC vs. systemic administration [
29], of PIPAC vs. intraperitoneal liquid chemotherapy [
29], of PIPAC vs. HIPEC [
86] and of PIPAC vs. laparoscopic HIPEC [
30]. In the swine model, tissue delivery of oxaliplatin was compared between laparoscopic HIPEC (with 12–15 mmHg pressure), PIPAC (20% of HIPEC dose applied) and electrostatic precipitation PIPAC (ePIPAC, same dose as PIPAC): overall concentrations in the peritoneum were not different among the three groups, documenting an improvement of the therapeutic index (target tissue dose/dose applied) of PIPAC vs. HIPEC by a factor of five. In the visceral peritoneum, the improvement of the therapeutic index reached a factor of 7.4 in favor of PIPAC (
p = 0.02).
3.4. Depth of Tissue Penetration
Most studies on depth of tissue penetration have been performed with doxorubicin, a large auto-fluorescent molecule with a molecular weight of 543 g/mol [
91]. Doxorubicin only penetrates a few cell layers after HIPEC [
92], corresponding to a depth of 10–30 µm. After PIPAC, depths of doxorubicin tissue penetration reached up to 469 µm in the ex vivo model [
84], more than 300 µm in the small intestine of swine post-mortem [
85] and up to 500 µm in human patients [
58].
3.5. Local Effect vs. Systemic Uptake
Since the dose applied is reduced by an order of magnitude (as compared to a usual systemic dose) [
93] and due to the properties of the peritoneum–plasma barrier, the plasma concentration of the chemotherapeutic agent remains low [
57] and, accordingly, the organ toxicity [
70]. In addition, pressure enhances the pharmacokinetic advantage of regional delivery by reducing blood outflow from the abdomen over the liver and the abdominal wall during the uptake phase. Thus, during the time of PIPAC exposition (currently around 30 min) at a pressure of 12–15 mmHg, the abdominal vascular compartment is expected to be partially isolated from the systemic compartment, contributing to better local uptake and less systemic toxicity.
This hemodynamic effect of pressure during laparoscopy has been documented in the preclinical model and in the human patient. Specifically, in a swine model of laparoscopic nephrectomy at a pressure of 12 mmHg, hepatic perfusion (both arterial and portal) was reduced by half: a decrease of portal flow from 974 mL/min to 547 mL/min and a decrease of hepatic artery flow from 278 mL/min to 133 mL/min was seen [
94]. When splanchnic blood flow was measured at an increasing intraabdominal pressure from 0 mmHg to 15 mmHg in 18 patients undergoing routine laparoscopy, portal venous flow was decreased by 39% and microcapillary flow in the parietal peritoneum by 60% [
95]. In the swine model, systemic oxaliplatin concentrations were significantly lower during PIPAC application time (in the presence of an intraabdominal pressure of 12–15 mmHg) than in the laparoscopic HIPEC group (
p < 0.05) [
30]. In vitro, the rate of apoptotic and proliferative cells as well as the level of oxaliplatin penetration in tumor nodes was higher in PIPAC groups with less systemic passage through the peritoneum [
86]. In vivo, in a mouse model of colorectal PM, systemic passage was lower in the PIPAC group [
86].