Limbal epithelial stem cells (LESCs), which live in a specialized stem cell niche (SCN), are crucial for the survival of the human corneal epithelium. They live at the bottom of the limbal crypts, in a physically enclosed microenvironment with a number of neighboring niche cells. Scientists also simplified features of these diverse microenvironments for more analysis in situ by designing and recreating features of different SCNs.
- stem cell
- niches
- microenvironment
- cornea
1. Introduction
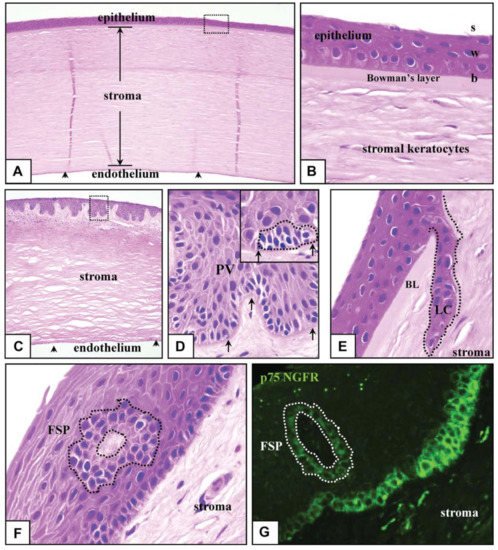
The cornea is made up of nonkeratinizing squamous epithelium, avascular, collagen-rich epithelial cells that are formed by self-renewing, stratified tissues [3]. The cornea’s transparency is crucial and primarily due to unique characteristics of the corneal stroma. The lack of blood vessels, the distinct organization of collagen fibers and the low percentage of stromal cells are all essential features in this regard [4]. The corneal epithelium lines the stromal surface and defends it from chemical insults. It is also important for the preservation of the stroma’s transparency-enabling properties. Moreover, with the exception of keratinizing epithelia, such as the epidermis, which replaces the cytoplasm of the outer layers with keratin proteins, the corneal epithelium keeps live cells at the edge layer, enhancing transparency.
The epithelium layer of the cornea acts as a protective and defensive shield, while also contributing to corneal openness. It is constantly switched over when the most superficial cells of the ocular epithelium fade away and are replaced by limbal epithelial stem cells (LESCs). LESCs are initiated from the limbus region, which is the border between the conjunctiva and cornea [5]. LESCs rely on their unique microenvironment, identified as the limbal niche, for separation, growth and movement. Cells such as mesenchymal cells, nerve cells, melanocytes, skin cells and vascular cells, ECM (extracellular matrix) and signaling molecules distinguish the limbal niche [6,7,8,9,10,11]. Pathology affecting any part of the limbal niche can cause LESC disorder, which leads to successful LSCD (limbal stem cell deficiency) [9,12,13].
The recent understanding of CESC biology has a special emphasis on the development of the stromal microenvironment, or niche, reconstruction of the LSCN (limbal stem cell niche), ASCN (artificial stem cell niche) and in regulating stem cell function.
2. Human Cornea
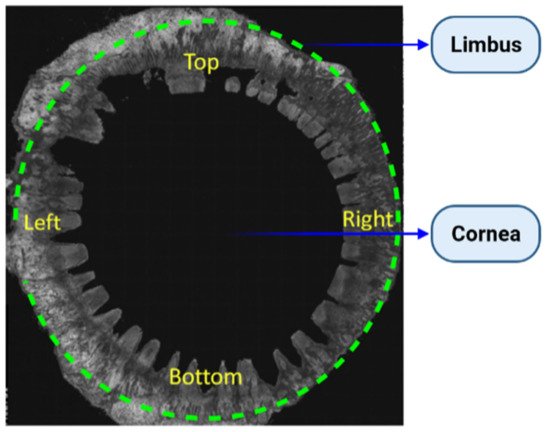
3. Identity and Location of CESCs (Corneal Epithelial Stem Cells)
For several years, the position of CESCs has been intensively studied, and it is still a very involved and rather contentious field of study. The prevalent and commonly accepted model holds that CESCs are only found at the limbus, which is located at the intersection of the conjunctiva and the cornea. This is supported by data from a number of tests. To begin, epithelial cells in the limbal epithelium’s basal layer feature is especially useful for young, undifferentiated cells, which are consistent with the existence of SCs (stem cells). In particular, epithelial cells in this region lack expression of cytokeratins 3 and 12, that are produced by adult, separated corneal epithelial cells, but maintain expression of cytokeratin 14, that is produced by immature stem or progenitor cells in the basal cell layer of a range of stratified epithelia. Moreover, several cells in the limbus possess putative stem cell receptors. They contain the N isoform of p63, that is represented by progenitor cells or proliferative stem cells in many stratified epithelia, and the transmitter protein ABCG2, which imparts that this is a ‘side-population’ phenotype and is often thought to be a common stem cell indicator [1,3,33]. Some putative stem cell indicators produced by cells in this area contain N-cadherin and Fzd7 [33]. Moreover, the limbal epithelium comprises a higher percentage of quiescent cells that barely differentiate, a characteristic shared by lengthy stem cells in a number of other tissues [34].
The most compelling evidence suggesting the existence of stem cells in the limbus is the indication that cells derived from this area can readily produce long-term cell proliferation clones in vitro and can reconstruct the conjunctiva based on transplanting. Evidently, the clinical utilization of stem cells derived from the limbus demonstrates their clinical effectiveness, when they are utilized to repair the conjunctiva in patients who have sustained major damage to the corneal layer as a result of disease or injury [3,35].
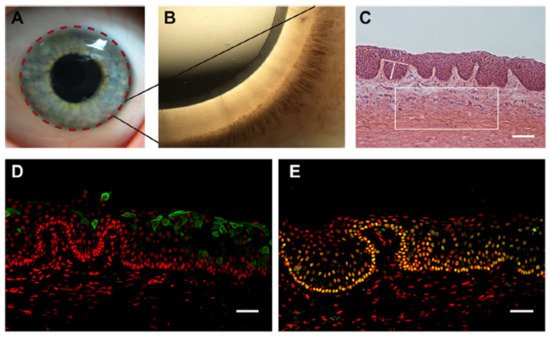
LSCNs (limbal stem cell niches) have been discovered in the limbus, as indicated in Figure 3A, especially in the palisades of Vogt, which are 150-square-meter structures [36]. The Palisades of Vogt have been described as anatomical stromal crypt structures that are particularly noticeable in some intact corneas due to the abundance of melanocytes, which are highly pigmented, as shown in Figure 3B. Separating fixed limbal tissue tangential to the central cornea and staining with H and E revealed stromal protrusions, which establish crypt-like structures that allow for the formation of cell layers in certain areas, as represented in Figure 3C. Longevity, high capacity for self-renewal with a long cell cycle period and short S-phase length, increased potential for error-free proliferation and poor differentiation are all characteristics of SCs.
4. The Corneal Stem Cell Niche
In order to maintain tissue equilibrium, the behavior of stem cells in every environment must be closely controlled. In this regard, the tissue niche or microenvironment, in which stem cells live, is crucial in controlling stem cell important factors when deciding [43]. The SCN (stem cell niche) is a specialized, different anatomical area of a tissue that provides the necessary microenvironmental clues to sustain a cell population capable of satisfying the tissue regeneration requirements of a tissue during any particular time. The crypt core of the small intestine, which contains intestinal epithelial SCs [44], and the bulge area of skin cells, which contains cutaneous epithelial SCs, are also representations of SCNs [40].
The limbus has some characteristics that differentiate it from the conjunctiva and the cornea on the human ocular surface. Perhaps most dramatically, the stromal tissue in the limbus produces papillae-like invaginations, recognized as the ‘Palisades of Vogt,’ and in between them are limbal epithelial crypts. Inside these crypt systems, a high percentage of basal epithelial cells produce putative SC indicators, such as Fzd7, N-cadherin and ABCG2, reinforcing the idea that the limbus provides a specialized stromal ecosystem designed to support CESCs.
The material characteristics of the underlying tissue have been seen to be critical in controlling SC activity. Topography and elasticity, for example, are also used to affect how a cell responds to other microenvironmental signals, including growth factors and/or cytokines [48]. In this respect, SCNs frequently have a specific topography and are made up of complex ECM elements, including one that endows the niche with unique mechanical properties [45,49]. The dome-like structure of the ocular surface is likely to exert distinct impact force at various areas of the tissue, which could favor SC repair at particular positions [38].
As a result, distinct limbal stromal features including such ECM structure, vascularization and growth factor function are critical in sustaining a functional population of CESCs. Analyzing how dermal ESCs are controlled by their microenvironment provides some insight into the molecular and cellular mechanisms for which niche elements control CESCs. Biochemical factors naturally produced by mesenchymal cells within the SCN are also used to regulate processes, including SC contemplation and stimulation in this tissue. Development of BMPs (bone morphogenetic proteins) by mesenchymal niche elements, for instance, enhances equanimity of dermal ESCs [52], while expression of fibroblast growth factors (FGFs), TGF and the BMP promoter noggin induces migration and cell growth [53,54]. Functional experiments in mice have also shown that the vasculature is critical in the stimulation of cutaneous ESCs, but the processes are still unknown. Other epithelial materials of the skin were shown to facilitate cell proliferation insertion in quiescent cutaneous ESCs by SHH (Secreting Sonic Hedgehog). Besides that, other materials found in the cutaneous SCN, including such ECM components, peripheral nerves and immune cells, were involved in SC activity regulation [49].
5. The Limbal Stem Cell Niche
The limbal stem cell niche, located at the anatomic boundary of the cornea and the conjunctiva [39,110,111], generates a microenvironment that aids in the growth and repair of their signals, resident cells and ECM, that identify a SCN [110,112]. Due to the occurrence of melanin pigmentation, the corneoscleral limbus has an identifiable protective atmosphere with thick protection, vascularization and innervation from possible light destruction [20,113,114]. The limbal palisade and corneal transformation areas tend to regulate cell proliferation sensing, creating a distinct microenvironment for progenitor cells and CESC. Though elements of the SC membrane in the dorsal limbus could provide external stimuli that lead to stemness repair [30,43,115,116,117], elements of the delayed progenitor cell membrane in the anterior limbus could control the phenotypic variations required to regenerate the restoring corneal epithelium [30].
While the presence of the limbal niche is acknowledged, particular details of its 3D architecture remain unknown [118]. Research of the construction of the limbal crypts and the corneal limbus has been performed utilizing various methods, with various mechanisms discovered [31,112,113,114,119,120,121,122]. Goldberg and Bron (1982) and Townsend (1991) utilized a pit light to observe the corneal limbus and identified the Vogt palisades as a set of circular patterns aligned fibrovascular ribs clustered along the superior and inferior corneoscleral limbus, differentiated by inter-palisade epithelial varied forms. They discovered a large variation of the palisade region from one human to the next and within the same eye, as well as a large diversity of the form of inter-palisade epithelial crypts and palisades, such as palisade branching, radially directed rectangular and/or circular or oval shapes, or connectivity to create a trabecular structure [113,114].
Microenvironment Structure

6. The Limbus and Other Stem Cell Niches
In the last five decades, it was suggested that a SCN offers a distinct and sufficient microenvironment for multipotential operation and to promote self-renewal [17]. Niches are 3D (three-dimensional) SC-sheltering, finely ordered, dynamic structural elements that are typically found at tissue boundaries, intersections or areas; for instance, endo-ectocervical, cornea-limbal and esophagogastric [18]. The differential clues that determine SC homeostatic or stimulation programs are assumed to be provided by molecular crosstalk from neighboring cells and reversible signals from the subsequent vasculature or ECM-sequestered intermediaries specific to the microenvironment. Chemical and physical signals between the 3D spaces’ cells and matrix glycoproteins they shape enable for intermolecular forces that are essential for controlling SC activity. The detection and classification of tissue niches has identified a constellation of materials; nevertheless, the processes governing how niches are formed and preserved to serve SC roles are only now being established [19]. Furthermore, new methods for marking SC in vivo have made it easier to identify and characterize SC niches in mammalian cells [1,2,19,141].
The hair follicle has appeared for one of the most thoroughly researched adult SC templates. Multipotent HFSCs (hair follicle stem cells) that regenerate skin, hair and sebaceous glands exist in the hair follicle bulge, an area of the basal part sheath. Previous studies that took to the benefits of bulge cells’ weak cycling allowed them to be identified and isolated as label-retaining progenitors [34,142]. When such cells were grafted into hairless mice, they developed huge colonies in situ and unchanged hair follicles. The identification of molecular techniques to help classify HFSCs has greatly enhanced scientists’ and researcher’s knowledge of their anatomy and biology, such as their adhesion to ECM proteins and the molecules related to cell regulation.
The pathways involved in regulating adult HSCs (hematopoietic stem cells), such as the HFSC niche, are widely undefined. HSCs are a type of bone marrow-derived cell that can self-renew or differentiate into several types of cells. HSCs that are dormant cross the inner layer of bone filled with osteoblasts [143]. When HSCs reach maturity, they cross paths with the stromal cells around them and continue to propagate. Multiple studies containing osteoblast-ablated mice and mice bioengineered to enhance osteoblast number have led to one regulatory hypothesis, which indicates that HSCs maintain their quiescent features owing to their ability to bind to osteoblasts via N-cadherin-mediated adherent intersections [143,144].
The cornea’s clarity sets it apart from other SC-containing organs. In addition, owing to its shallow anatomical position, the limbus seems to be the only SCN that can be easily observed utilizing noninvasive small-hole and in situ imaging techniques. The widely held belief is that unipotent LSCs within the basal cells of the limbus preserve the ocular epithelial cells through natural cellular proliferation and after injury [22,23,147]. Studies demonstrating the movement route of pigmented cells from the limbal region [20] provide insights as to the position of cells with regenerative potential.
7. Conclusions and Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering8080108
