Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
In epileptic patients, pharmacological treatment with available anticonvulsants leads to seizure control in <70% of cases. Surgical intervention can lead to control in a selected subset of patients, but still leaves a significant number of patients with uncontrolled seizures. In drug-resistant epilepsy, the ketogenic diet proves to be useful.
- epilepsy
- drug-resistant epilepsy
- ketogenic diet
- therapy
- ketones
- gut microbiota
- side effects
1. Introduction
Epilepsy is a chronic brain disorder that is characterized by recurrent seizures, which are short episodes of involuntary movement that can affect part or all of the body, sometimes accompanied by loss of consciousness and control of bladder or bowel function. Epilepsy is defined as the occurrence of 2 or more unprovoked seizures. A common type of epilepsy affecting 6 in 10 people is idiopathic epilepsy, which means that in over 50% of global cases, the cause of the disease is not identified [1]. Epilepsy of known cause is called secondary or symptomatic epilepsy. Causes of secondary or symptomatic epilepsy are: brain tumors, stroke, brain infection and severe head injury, congenital abnormalities associated with brain defects, brain damage as a result of prenatal or perinatal injuries, and certain genetic syndromes [2]. About 50–70 million people worldwide suffer from epilepsy [2,3]. It is estimated that 2.4–4.6 million people worldwide are diagnosed with epilepsy each year [3]. These global load estimates are falling more on the populations of low- and middle-income countries, where the cumulative estimate of annual incidence of epilepsy is much higher (139 per 100,000 people) than in high-income countries (49 per 100,000 people) [3]. Regardless of the country’s income, the public health burden of epilepsy carries a high risk of disability, economic loss, social isolation, and premature death [4]. Epilepsy is a serious and costly health problem worldwide and includes estimated indirect and direct costs annually of around EUR 15.5 billion in Europe [4] and USD 15.5 billion in the United States [5]. In this regard, the World Health Organization has made this a priority, calling for the development of national healthcare plans for the treatment of epilepsy, not only to ensure the availability of effective care, but also to prevent its causes. Almost 80–90% of people diagnosed with epilepsy live in low- and middle-income countries [2,6]. Recent studies in low-, middle-, and high-income countries have shown that up to 70% of adults and children with epilepsy can be successfully treated with antiepileptic drugs. After 2 to 5 years of successful therapy and no seizures, medications can be withdrawn in approximately 70% of children and 60% of adults without recurrence. As the above information shows, available pharmacological treatment for epilepsy has limited effectiveness. Surgical intervention can lead to seizure control in a selected subset of patients, but still leaves a significant number of patients with uncontrolled seizures. The ketogenic diet has proven useful in cases of epilepsy in which pharmacological and/or surgical treatment is not effective as shown below.
2. Classic Epilepsy Therapy
Classic epilepsy treatment includes pharmacological and surgical therapy or vagus nerve stimulation. Despite these therapies, approximately 30% of patients with epilepsy do not have sufficiently controlled seizures and become resistant to drugs [7]. This is defined as insufficient seizure control, despite optimal therapy using a combination of two or more appropriately selected antiepileptic drugs. Under these circumstances, adding next antiepileptic drug often does not significantly reduce seizures. Although epilepsy research is ongoing, the mechanisms of this disease have not been completely elucidated and fully effective therapy for all epilepsy patients has not yet been developed. Epilepsy is the highest research priority for many pharmaceutical companies, which makes epilepsy one of the most studied brain disease in the pharmaceutical industry, but despite such tremendous commitment, we are not seeing significant progress in developing new effective drugs. Patients with drug-resistant epilepsy are addicted to informal care of family and friends as well as healthcare professionals such as social workers, neurologists, and psychologists. Problems associated with drug-resistant epilepsy in children, adolescents, and adults cause repeated hospitalizations of numerous patients. Living with uncontrolled epilepsy has a negative impact on the quality of life of patients with epilepsy and their caregivers.
3. Ketogenic Diet
A ketogenic diet should be considered for patients who have not responded adequately to therapy with two well-selected and well-dosed antiepileptic drugs. Therefore, neurologists often recommend other therapies, such as diet, including ketogenic diet, to provide patients with better antiepileptic control. The ketogenic diet is a last resort treatment for many children, adolescents, and adults with epilepsy resistant to routine medications. It should be recognized that, despite the development of new antiepileptic drugs every year, the treatment, as already mentioned, in about one-third of patients with epilepsy is not fully effective. Ketogenic diet treatment is a non-pharmacological therapy used worldwide, especially for children with epilepsy that is difficult to control. Ketogenic diet has been used in patients with difficult-to-treat epilepsy since 1921, with minor changes in recent years [7]. The ketogenic diet assumes a very high-fat and low-carbohydrate diet, reducing carbohydrate to less as 10% of used energy [8]. This restriction triggers a systemic shift from glucose metabolism toward the metabolism of fatty acids yielding ketone bodies, such as acetoacetate and β-hydroxybutyrate as substrates for energy. The ketogenic diet provides sufficient protein for growth and development. Energy is mostly derived from fat delivered in the diet and by the utilization of body fat. The ketogenic diet is a biochemical model of fasting, which shifts organs to utilize ketone bodies as the source to replace glucose for the brain. The ketogenic diet allows about 90% of the total caloric income from fat and 6% from protein and 4% from carbohydrates. For many refractory epileptic patients, dietary treatment promises to improve the quality of life with a significant decrease in seizure frequency. For this reason, an increase in the global use of the ketogenic diet is currently observed. Successful implementation of this diet depends on the active support of the health care team, the social and educational system, and finally the family. The ketogenic diet requires strict dietary and medical control due to its restrictiveness and side effects [6,7].
4. Possible Anti-Seizure Mechanisms of the Ketogenic Diet
Although the anticonvulsant mechanisms of ketogenic diet are not still completely understood, it is believed that ketone bodies and polyunsaturated fatty acids presumably play a major role in the anticonvulsant effect of ketogenic diet. During ketogenic diet treatment, body energy is generally generated by the oxidation of fatty acids in mitochondria, resulting in the production of large amounts of acetyl-CoA. Accumulation of acetyl-CoA leads to the synthesis of two ketone bodies mainly in the liver, acetoacetate, and β-hydroxybutyrate, which then enter the blood circulation. Ketone bodies are then used as an alternative source of energy in the brain instead of glucose. After entering the brain, the ketone bodies are transformed into acetyl-CoA and then enter the tricarboxylic acid cycle in the mitochondria of the brain, which ultimately leads to the production of adenosine triphosphate (ATP). Several hypotheses regarding ketone bodies are considered as key mediators involved in the anticonvulsant effect of the ketogenic diet. Based on several studies, potential mechanisms focus essentially on the role of neurotransmitters, brain energy metabolism, oxidative stress, and ion channels, which are briefly discussed below [23,24] (Figure 1).
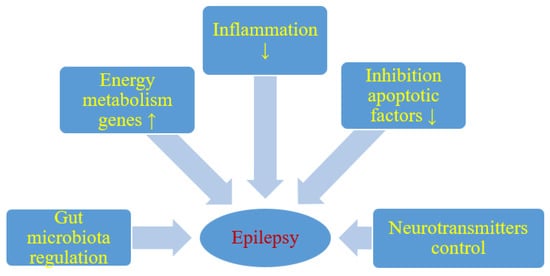
Figure 1. Likely effect of a ketogenic diet on seizure activity. ↑- increase, ↓- decrease.
It has been shown that energy production in the brain is significantly increased by ketogenic diet. Long-term ketogenic diet therapy increases the expression of energy metabolism genes, improves mitochondrial biogenesis and density, and increases energy reserves in the form of phosphocreatine [24] (Figure 1). This improves the function of neurons and increases their chances of surviving in stressful conditions. It is believed that brain tissue under the influence of a ketogenic diet becomes more resistant to metabolic stress, and this increases the seizure threshold. Under the conditions of a ketogenic diet, a decrease in brain glucose consumption and the production of glycolytic ATP may induce potassium channels sensitive to ATP opening, which leads to hyperpolarization of the neuronal membrane. This reduces electrical excitability of the brain and increases the seizure threshold. Additionally this prevents excessive firing of neurons and regulates the seizure threshold in the brain. It is also suggested that two-pore domain potassium channels may also be activated by ketone bodies and some fatty acids. Thus, a ketogenic diet triggered increase in the blood ketone bodies and fatty acids may also regulate neuronal membrane excitability by activating two-pore domain potassium channels, and this can be taken as another likely anticonvulsant mechanism of the ketogenic diet.
Reduction of neuronal excitability is the most important role of GABA in the brain and therefore GABA plays a key role in the initiation and spread of seizure activity in the brain. It has been observed that ketogenic diet can lead to glutamic acid decarboxylase activation, which induces GABA synthesis [23]. It has also been shown that this diet can alter GABA transaminase activity that inhibits GABA degradation. Increasing energy metabolism through a ketogenic diet can compensate for the metabolic and transient failure of GABAergic inhibition, the lack of which will not prevent the occurrence and spread of seizures. Therefore, another important mechanism induced by the ketogenic diet in anticonvulsant activity is probably mediated by the GABAergic system [23] (Figure 1).
High levels of glutamate in the brain can make the brain more susceptible to seizures and therefore glutamate is associated with the development of epilepsy. The results of studies on the effect of the ketogenic diet on glutamate level are inconclusive [24], it has been shown that this diet can increase the level of glutamate in the brain synaptosomes, while other studies showed no effect [23,25].
Agmatine has been found in synapses and can be considered as an inhibitory neurotransmitter. It may exert an anti-seizure effect, probably by inhibiting various brain stimulating receptors, including N-methyl-D-aspartate, histamine, and adrenaline receptors. It has been shown in rat studies that ketogenic diet can increase the level of agmatine in the hippocampus [23] (Figure 1). The above observations support the view that the ketogenic diet increases the level of agmatine in the brain, which has neuroprotective properties, therefore these properties can be considered as another anticonvulsant mechanism of the ketogenic diet [23]. Additionally, agmatine may potentiate the anticonvulsant action of valproate and phenobarbital against maximal electroconvulsions in mice with no pharmacokinetic interaction involved [26]. In another model of seizures induced by pentylenetetrazol in mice, agmatine was documented to attenuate the protective activity of vigabatrin, other numerous antiepileptic drugs being not affected [27]. If agmatine is involved in the mechanism of the ketogenic diet in clinical conditions, then some positive or negative outcomes may be observed when combined with particular antiepileptic drugs.
It has been noted that monoamine neurotransmitters—including noradrenaline, dopamine, and serotonin—play an important role in controlling the excitability of neurons and seizures [28,29]. Many neural networks have been shown to have serotonin and dopamine receptors that are involved in seizures. It has been shown that in animals without a functional noradrenergic system, the ketogenic diet did not show anticonvulsant capacity [24]. In addition, it has also been shown that the levels of serotonin and dopamine in the cerebrospinal fluid may be affected by ketogenic diet in children with drug-resistant epilepsy [29].
In addition, it appears that particularly polyunsaturated fatty acids provided by the ketogenic diet may activate peroxisome proliferator-activated receptors that regulate anti-inflammatory, antioxidant, and mitochondrial genes leading to increased energy reserves, stabilization of synaptic functions and restriction of hyperexcitability [30] (Figure 1).
It has been revealed that single small convulsions are not able to kill neurons, while severe long-term seizures can not only cause neuronal damage but also their death [24,31]. Cognitive impairment and severity of seizures in patients with drug-resistant epilepsy may depend on the degree of neuronal damage and death caused by seizures [32]. Everything indicates that excitotoxicity and apoptosis are the main mechanisms involved in seizure-related neuronal damage and death. It has been suggested that the negative consequences of these neuropathological processes can be improved by means of a ketogenic diet [33] (Figure 1). It has been observed that ketogenic diet can upregulate calbindin which has neuroprotective potential through its ability to buffer intracellular calcium [34]. Other neuroprotective properties of the ketogenic diet may mediate the inhibition of apoptotic factors such as caspase 3 [34,35] (Figure 1). Opening transient pores in mitochondria can also be inhibited by a ketogenic diet [36].
5. Ketogenic Diet and Gut Microbiota: Friends or Foes?
Dysbiosis may be involved in the drug-resistant epilepsy mechanism, and restoration of intestinal microbes may be a new therapeutic method in drug-resistant epilepsy [37,38,39] (Figure 1). People with drug-resistant epilepsy show altered intestinal microflora [37,38]. Numerous rare flora increases in patients with drug-resistant epilepsy [38]. Then Hampton [37] suggested that the antiepileptic effect of the ketogenic diet could be attributed to intestinal microbes (Figure 1).
The mechanisms by which the ketogenic diet exerts an anticonvulsant effect are likely to be numerous and may vary in different types of epilepsy. Recent articles describe a new mechanism for ketogenic diet to prevent seizures by changing gut microbiota in animals and humans [40,41,42]. To date, very few studies have focused on the role of gut microbiota in the treatment of epilepsy using a ketogenic diet [40,41,42,43]. Olson et al. [40], presented very interesting research on gut microbiota-dependent anticonvulsant properties of the ketogenic diet in which two mouse models of refractory epilepsy were used, demonstrating the relationship between the ketogenic diet and gut microbiota to obtain a therapeutic effect. Diet significantly increases the relative abundance of Akkermansia muciniphila, from 2.8% to 36.3% during 4 and 14 days of dietary treatment. Parabacteroides merdae, Sutterella, and Erysipelotrichaceae also increased significantly, while Allobaculum, Bifidobacterium, and Desulfovibrio were lower in mice fed the ketogenic diet compared to mice fed the control diet. Akkermansia muciniphila and Parabacteroides merdae have been shown both to be necessary to achieve the anti-seizure effect of a ketogenic diet. The combination of these two bacterial taxa restored protection against seizures in antibiotic-treated mice after administration of the ketogenic diet. On the contrary, colonization with only Akkermansia muciniphila or Parabacteroides distasonis did not protect against seizures and there was no significant increase in seizure threshold. Similarly, colonization of Akkermansia muciniphila and Parabacteroides together, but not separately, protected against seizures in germ-free mice fed a ketogenic diet [40].
This entry is adapted from the peer-reviewed paper 10.3390/nu11102510
This entry is offline, you can click here to edit this entry!
