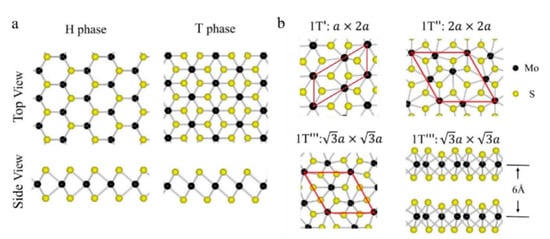
MoS2 is one of the transition metal dichalcogenides (TMDs) that has gained a high reputation in recent years due to its distinct chemical, electronic, mechanical, magnetic, and optical properties. Its unique properties enabled its use in different applications such as sensing applications, high-efficiency field effect transistors, and energy and medical (curing) applications. MoS2 exists in different crystalline structures, such as hexagonal (H), tetrahedral (T), or rhombohedral (R). It naturally exists as 2H MoS2, and its most popular structures are the semiconducting 2H and 3R phases and the 1T metallic phase, where 2H is more stable but less conductive than 1T. Metallic MoS2 has a higher conductivity (105 times) than semiconducting 2H MoS2 and high catalytic activity.
- 二硫化钼
- 二硫化钼能源应用
- 太阳能电池
- 析氢反应 (HER)
- 金属二硫化钼
- 1T 二硫化钼
1. Structure and Properties
2. Energy Applications
2.1. Energy Storage Applications
2.1.1. Lithium-Ion Batteries (LIB)
| Battery Type | MoS2 Phase | Structure | Capacity | References |
|---|---|---|---|---|
| Lithium-ion | 1T (Metallic) | Nanotube-like MoS2 over graphene | Discharge capacity = 666 mA h g−1 at current density = 3500 mA g−1 |
[61] |
| Lithium-ion | 1T (Metallic) | MoS2 over carbon cloth | Reversible specific capacity = 1789 mA h g−1 at 0.1 Ag−1 Retained capacity = 853 mA h g−1 after 140 cycles at 1 Ag−1 |
[63] |
| Lithium-ion | 1T (Metallic) | 1T MoS2 + (NiMoO4) | Charged mass capacity = 940.1 mA h g−1 Discharged mass capacity = 941.6 mA h g−1 |
[74] |
| Lithium-ion | 1T (Metallic) | Pure MoS2 | Specific capacity ≈ 935 mA h g−1 for 200 cycles at 5 A g−1 can be increased to 1150 mA h g−1 |
[62] |
| Sodium-ion | 1T (Metallic) | MoS2-graphene-MoS2 | Capacity of 175 mA h g−1 at a high current density of 2 A g−1 Reverse capacity of ≈313 mA h g−1 at low current density of 50 mA g−1. Stabilizes at current density = 313 mA h g−1 after 200 cycles |
[43] |
| Sodium-ion | 2H and 1T MoS2 | Dual phase of 2H and 1T MoS2 | Capacity = 300 mA h g−1 after 200 cycles, and coulombic efficiency = 99% |
[75] |
| Sodium-ion | 2H phase transfers to 1T through chemical reactions | MoS2 and amorphous carbon (C) | Capacity = 563.5 mA h g−1 at 0.2 A g−1 Coulombic efficiency = 86.6% Cyclic stability = 484.9 mA h g−1 at 2 A g−1 |
[76] |
| Supercapacitor | 2D MoS2 | Spraying MoS2 nanosheets on Si/SiO2 | Area capacitance = 8 mF cm−2, and volumetric capacitance = 178 F cm−3 |
[77] |
| Supercapacitor | Nanoflower-like MoS2 structure | 3D-graphene/MoS2 nanohybrid | Dimensions 23.6 × 22.4 × 0.6 mm3 Specific capacitance (Csp) = 58 F g−1, energy density of 24.59 W h Kg−1, and power density of 8.8 W Kg−1 with operating window of 2.7 V (−1.5 to +1.2 V) |
[78] |
| Supercapacitor | Brush-like arrangement MoS2 | MoS2 nanowires over Ni foam | The high mass loading of MoS2 (30 mg cm−2) retains 92% of maximum capacitance after 9000 charge–discharge cycles at 5 A g−1 | [79] |
| Supercapacitor | MoS2 QSs | Exfoliated MoS2 QSs lateral size (5–10 nm) | Capacitance = 162 F g−1 Energy density = 14.4 W h kg−1 |
[80] |
| Hybrid Supercapacitor |
N-3DG and 3D-IEMoS2@G |
Prepared using solvothermal process | Energy density = 140 W h kg−1 at 630 W kg−1, and 43 W h kg−1 at power density of 103 kW kg−1 Lifecycle over 10,000 |
[81] |
2.1.2. Sodium-Ion Batteries (NIB)
2.1.3. Supercapacitors
2.2. Energy Generation Applications
2.2.1. Hydrogen Evolution Reactions (HER)
| Type of Reaction | Catalyst Used | Specification | References |
|---|---|---|---|
| HER | (MoS2/CoSe2) | Tafel slope = 36 mV dec−1 Onset potential = −11 mV Exchange current density = 7.3 × 10−2 mA cm−2 |
[95] |
| HER | 1T MoS2 | Overpotential = 156 mV, at 10 mA cm−2 Tafel slope = 42.7 mV dec−1 |
[96] |
| HER/OER | Amorphous Ni–Co complexes hybridized with 1T MoS2 | Overpotentials = 70 mV HER and 235 mV for OER at 10 mA cm−2 Tafel slope = 38.1 to 45.7 mV dec−1 |
[97] |
| OER | Rhombohedral MoS2 microspheres over conductive Ni | Overpotential ≈ 310 mV Tafel slope ≈ 105 mV dec−1 |
[98] |
| OER | MoS2 quantum dots (MSQDs) | Overpotential = 280 mV Tafel slope = 39 mV dec−1 |
[99] |
| CO2 reduction | Vertically aligned MoS2 nanoflakes (2H and 1T phases coexist) |
Overpotential = 54 mV Reduction current density = 130 mA cm−2 at −0.764 V |
[100] |
| CO2 reduction | p–n junction Bi2S3/MoS2 composite |
Photocatalytic CO2 reduction 20 times higher than single catalysts under visible light irradiation |
[101] |
| CO2 reduction | 3R MoS2 nanoflower powder | Synthesized using CVD CO production < 0.01 μmol-gcat−1 hr−1 at 25 °C which is negligible |
[102] |
2.2.2. Oxygen Evolution Reactions (OER)
This entry is adapted from the peer-reviewed paper 10.3390/en14154586
