Extracorporeal membrane oxygenation (ECMO) is increasingly used for acute respiratory failure with few absolute but many relative contraindications. The contraindications to the initiation of ECMO therapy are not uniformly agreed upon, and each center, as well as each provider involved in the indication for the initiation of ECMO, weights them differently. Whereas absolute contraindications immediately discourage ECMO therapy, relative contraindications should trigger a very thorough consideration of this option. Although relative contraindications should not per se exclude patients from a life-saving procedure such as ECMO, their concurrence may lead to the decision to forgo this procedure. When relative contraindications add up, they might accumulate to a point where they (should) be considered absolute contraindications.
1. Indications for the Initiation of ECMO
Although contraindications to the use of ECMO are largely relative and increasingly questioned, indications for the use of ECMO are widely agreed upon.
Currently almost universally used as rescue-therapy, evidence is accumulating that ECMO treatment could be even more beneficial in ARDS if instituted early, i.e., before the injurious effects of conservative treatment (i.e., ventilator induced lung-injury (VILI) or patient self-inflicted lung injury (P-SILI)) develop [
16].
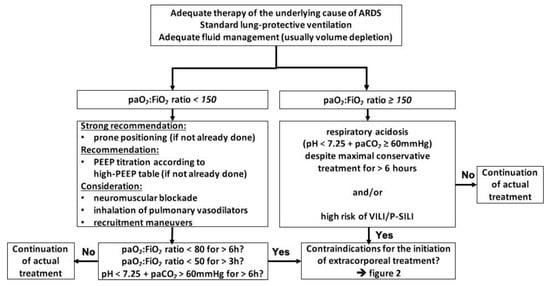
Currently, ECMO is most likely to be initiated by the majority of centers only if the following basic measures have been performed: the underlying cause of ARDS is identified and adequately treated, the patient is adequately resuscitated, which in most cases of ARDS means volume depleted, and the use of lung-protective ventilation strategies was implemented. If the paO
2:FiO
2 ratio remains below 150, prone positioning and ideally personalized adjustment of PEEP toward higher levels [
17] should be established. If these measures and possibly less beneficial rescue therapies such as neuromuscular blockade or NO inhalation [
18] were used and the paO
2:FiO
2 ratio remains below 80 for 6 h, below 50 for 3 h, respectively, the initiation of ECMO is indicated. The same applies for respiratory acidosis with a pH less than 7.25 sustained for more than 6 h despite maximal conservative treatment regardless of the paO
2:FiO
2 ratio [
19] (
Figure 1).
Figure 1. Algorithm to indicate ECMO initiation; adapted from [
19], modified from [
1,
5,
20]. Abbreviations: ARDS = acute respiratory distress syndrome, paO
2 = partial arterial pressure of oxygen, FiO
2 = inspiratory oxygen fraction, paCO
2 = partial arterial pressure of carbon dioxide, PEEP = positive end-expiratory pressure, VILI = ventilator-induced lung injury, P-SILI = patient self-inflicted lung injury.
Although the rule to exhaust conservative strategies before considering ECMO is not always consistently followed, and the algorithms and decision trees used to determine the failure of these measures vary, they are generally well accepted.
2. Absolute Contraindications to the Initiation of ECMO
2.1. Refusal of the Use of Extracorporeal Techniques by the Patient
Refusal of possible therapy is a fundamental right of a person of sound mind. It can be expressed in the form of stock phrases in an advance directive, an actually expressed patient will, or a presumed/actual patient will by relatives/legal representatives in the case of a currently incapacitated person. The refusal of a possibly life-saving therapy must be respected if the person is aware of the implications of the decision to refuse treatment. However, caution must be exercised when this refusal is expressed by the next of kin/legal representatives. If there is even the slightest doubt about the legitimacy of this refusal, it is best to err on the side of therapy, with the option of discontinuing any therapy initiated at an early stage [
21].
2.2. Advanced Stage of Cancer
No studies were identified that explicitly reported the effect of vvECMO on outcome in advanced cancer stages; thus, we attempted to make an analogy.
The principle “primum nil nocere” has been handed down from the beginnings of medicine; this paradigm is still relevant, perhaps even more so in modern intensive care medicine with its seemingly infinite measures and treatment options. It is the responsibility of the bedside physician not to initiate treatment that is unlikely to succeed in the broadest sense. The reduced life expectancy due to an advanced stage of cancer represents one of those circumstances in which ethical and medical triage can confluence. Although life expectancy cannot be reliably predicted today, advanced stages of cancer are generally associated with a reduced life expectancy, and treatments to prolong life in these stages are usually rejected. However, ECMO has been reported to facilitate palliative interventions [
22,
23,
24,
25].
Treatment goals in these patients require a very clear definition. Apart from a fundamental discussion of economic burden and distributive justice, initiating ECMO therapy for a limited period of time, to achieve a prespecified treatment goal, can be justified for individual patients.
Individual net gain for a patient burdened with end-stage cancer needs a thorough evaluation. We believe that patients facing a life expectancy of less than 5 months should not spend them in an ICU supported by an ECMO but rather in a palliative setting with symptom control close to family and friends. It is important to note, however, that assessment and attitudes of patients, relatives, and healthcare providers vary in this regard.
2.3. Fatal Intracerebral Hemorrhage/Cerebral Herniation/Intractable Intracranial Hypertension
Supratentorial mass lesions due to various reasons (e.g., intracranial hemorrhage (ICH), tumors, and cerebral edema as a consequence of traumatic brain injury (TBI)) can result in fatal transtentorial herniation. Rapid expansion of a supratentorial mass displaces the temporal lobe medially, after which the Uncus herniates over the edge of the tentorium cerebri; further progression leads to impaction of the temporal lobe in the tentorial notch, resulting in fatal secondary ischemia of the brainstem [
26]. To disrupt this progression, medical and surgical measures have been advocated for decades. In
Table 1, the literature from the last 3 decades is summarized with regard to mortality, as well as functional outcome of intracranial hypertensive conditions. There is a paucity of literature on ECMO in these populations [
22], therefore, we tried to establish an analogy.
Table 1. Outcome of different intracranial pathologies leading to intracranial hypertension. Abbreviations: GOS = Glasgow Outcome Scale, SDH = subdural hematoma, EDH = epidural hematoma, ICU = intensive care unit, TBI = traumatic brain injury, DC = decompressive craniectomy, ICH = intracranial hemorrhage.
| Study |
Pathological Condition |
Mortality |
Worse Outcome
(GOS ≤ 3 or Equal) |
| Gurer et al. 2017 [24] |
SDH/EDH |
49.1% (ICU) |
66.7% (6 months) |
| Gower et al. 1988 [27] |
swelling after TBI |
23% (ICU) |
60% (≥ 2 years) |
| Gaab et al. 1990 [28] |
swelling after TBI |
14% (ICU) |
22% (n/a) |
| Polin et al. 1997 [29] |
swelling after TBI |
23% (hospital) |
63% (discharge) |
| De Luca et al. 2000 [30] |
swelling after TBI |
18% (n/a) |
59% (n/a) |
| Taylor et al. 2001 [31] |
swelling after TBI (children) |
DC: 33% (1 week) medical: 42% (1 week) |
46% (6 months) |
| Whitfield et al. 2001 [32] |
swelling after TBI |
23% (10 months) |
31% (10 months) |
| Schneider et al. 2002 [33] |
swelling after TBI |
22.5% (6 months) |
71% (6 months) |
| Albanèse et al. 2003 [34] |
swelling after TBI |
early DC: 52% (1 year) late DC: 23% |
62% (1 year) |
| Aarabi et al. 2006 [35] |
swelling after TBI |
32.4% (30 days) |
48.7% (30 days) |
| Wettervik et al. 2018 [36] |
swelling after TBI |
DC: 17% (6 months)
Thiopental: 4%
no specific treatment: 11% |
DC: 60% (6 months)
Thiopental: 48%
no specific treatment: 27% |
| Sakai et al. 1998 [37] |
cerebral infraction/malignant swelling |
33% (2 months) |
67% (2 months) |
| Qureshi et al. 2000 [38] |
medical reversal of supratentorial masses |
54% (hospital) |
46% (Barthel & Rankin)
(≥6 months) |
| Koenig et al. 2008 [39] |
medical reversal of transtentorial herniation |
67.6% (hospital) |
77% (GOS 4 & 5) |
| Skoglund et al. 2005 [40] |
transtentorial herniation after TBI |
26% (≥6 months) |
41% (≥6 months) |
| Kim et al. 2009 [41] |
DC for TBI/ICH/infarction |
TBI: 21.4% (6 months)
ICH: 25% (6 months)
Infarction: 60.9% (6 months) |
TBI: 42.9% (6 months)
ICH: 50% (6 months)
Infarction: 69.6% (6 months) |
| Lan et al. 2020 [42] |
DC for herniation after TBI |
30.4% (6 months) |
66% (6 months) |
| Delcourt et al. 2017 [43] |
ICH |
12% (90 days) |
45.4% (90 days) |
| Chen et al. 2019 [44] |
infratentorial ICH |
8% (90 days) |
28% (90 days) |
| Poon et al. 2014 [45] (metaanalysis) |
ICH |
46% (1 year) |
up to 24% (1 year) |
| Pinho et al. 2019 [46] (metaanalysis) |
ICH |
36.3% (1 year) |
n/a |
We found that mortality was relatively low depending on the primary pathology. However, more than 50% of survivors remain in a low to very low or even vegetative functional state (Table 1).
According to data presented in Table 1, we strongly advocate against initiation of ECMO for acute respiratory failure in cases of intractable/uncontrollable intracerebral hypertension and/or cerebral herniation, let alone fatal intracerebral hemorrhage.
2.4. Irreversible Destruction of the Lung Parenchyma without the Option of Transplantation
No studies reporting explicitly on the effect of vvECMO on this topic were available; therefore, we reviewed the literature on pulmonary fibrosis and present our assessment and experiences.
To effectively exchange gas, the lung parenchyma must be very delicate, making it very efficient but vulnerable at the same time. The mechanism that damages lung parenchyma is usually an inflammatory process either directly due to acute infection or secondary due to a chronic process such as inhalation of tobacco smoke or air pollution. The response to an injurious event depends on the type, intensity, and number of damaged cells. Following acute but limited superficial damage (e.g., airborne infection, irritants, airborne toxins) the epithelial lining of the respiratory tract can stimulate an effective regenerative cascade emanating from adjacent healthy epithelium [
47]. If, however, the tissue structure/structural integrity of the lung provided by the basal membrane that underlies the alveolar epithelium is damaged, tissue is repaired rather than regenerated. Repairing in this context results in replacement of the normal/functional tissue architecture with fibrous tissue, consistent with scarring [
48]. The same process is obviously true for mechanical damage (i.e., disruption) to lung tissue due to direct impact. Idiopathic pulmonary fibrosis is another entity that leads to irreversible destruction of lung parenchyma [
49]. Depending on the extent of scarring/fibrotic transformation of the lung parenchyma, gas exchange is affected to various degrees. Fibrotic scar tissue will not contribute to gas exchange and will not heal but progress. Therefore, impaired gas exchange due to fibrotic tissue transformation in conjunction with contraindications to lung transplantation represents an absolute contraindication to the initiation of vvECMO. However, there are situations where extensive pulmonary fibrosis is present, but gas exchange is not severely altered and patients do not experience severe restrictions. If this satisfactory lung function at baseline is worsened by acute infection, this situation could/should represent a relative contraindication in individual cases [
50,
51]. Unfortunately, no guidance is available on the extent of fibrosis still “acceptable” to initiate ECMO treatment, while the mode of rating the extent of fibrosis remains ambiguous.
Regular computed tomography of the lungs does not contribute fundamentally to the assessment of the extent and severity of fibrosis, even more so if it is not performed in inspiratory hold.
Histological evaluation of the lung parenchyma requires samples that, in order to be meaningful, are often difficult to obtain and inherently only represent a small section of the organ. In addition, the procedure of obtaining a specimen usually by forceps biopsy during a bronchoscopy puts the patient at risk of bleeding and a period of worsening gas exchange. Above all this, the natural course, as well as the outcome, is unpredictable, and disease activity is usually monitored clinically [
52].
Based on experience of the authors, the morphological extent of fibrosis is less important than the clinical baseline status of the patient before the onset of the acute situation; this is consistent with the current literature [
52]. Taking into account that pulmonary status can only reach the baseline level in the best cases, in our opinion, baseline pulmonary functional status is the single most important factor predicting patient-centered outcomes and should not be ignored in favor of an image impression.
2.5. Contraindications to Transplantation without the Option of Sufficient Lung Healing
Survival rates after lung transplantation have significantly improved in recent years, mainly due to improvements in donor selection, organ preservation, and management of postoperative complications [
53,
54]. However, in the context of respiratory failure with an ambiguous chance of sufficient lung healing to be removed from extracorporeal support and the existence of a contraindication (
Table 2) [
55], vvECMO should be avoided.
Table 2. Contraindications to lung transplantation (adapted from Weill 2018 [
55]).
| Absolute Contraindications |
Relative Contraindications |
| History of malignancy (<2–5 years disease free plus high risk of recurrence) |
Age >65 years plus low physiological reserve |
| Significant dysfunction of another major organ system (heart, liver, kidney, brain) |
Mechanical ventilation/extracorporeal life support |
| Uncorrected coronary artery disease |
Controlled coronary artery disease |
| Unstable medical condition |
Significant osteoporosis |
| Uncorrectable bleeding |
Extensive prior chest surgery |
| Poorly controlled infection/resistant microbes |
Colonization with resistant microbes |
| Inadequate social support |
Infectious liver cirrhosis |
| Severe thorax deformity |
HIV infection (unless treated adequately) |
| BMI ≥35 kg/m2 |
BMI 30–35 kg/m2 |
| Nonadherence to medical therapy (recent & history) |
Significant malnutrition |
| Inability to comply with therapy |
Specific infections [55] |
| Active tuberculosis/contraindications to immunosuppression |
Poorly controlled diabetes, hypertension, epilepsy, peptic ulcer disease, gastroesophageal reflux, or central venous obstruction |
| History of illicit substance abuse |
|
| Inability to participate in rehabilitation |
3. Relative Contraindications to the Initiation of ECMO
3.1. Advanced Age >70 Years
Age is a well-established risk factor for mortality in the ICU [
56,
57]. Due to an increasing proportion of old and very old people and an increasing life expectancy, more and more old and very old patients are admitted to an ICU with acute respiratory failure, potentially requiring extracorporeal life support [
58]. The outcome of elderly patients treated with ECMO support has been evaluated in several trials, all showing moderate to high mortality of 50% or more in patients 65 years and older (
Table 3). On the contrary, single case reports describe good survival and outcomes in patients 70 years and older [
59,
60]. Personal experience of the authors confirms both. We consider a mortality rate of 50–60% to not be an absolute contraindication for initiation of ECMO, particularly because different causes of ARDS vary in their mortality rates and should be taken into account. However, starting above 70 years, age should be seen as a relative contraindication. This could still be acceptable if the patient is otherwise in reasonable condition. However, if combined with an increasing number of relative contraindications such as those in the sections below, it should more and more be regarded an absolute contraindication in accumulation.
Table 3. Evidence for the use of vvECMO in elderly patients and the respective outcome.
| Study |
Age Defining
“Elderly” |
No. of Patients
Included Total |
Hospital Mortality in the “Elderly” |
| Mendiratta et al. 2014 [58] |
>65 |
368 |
59% |
| Karagiannidis et al. 2016 [8] |
>80 |
1944 |
76% |
| Deatrick et al. 2020 [61] |
>65
>55 |
182 |
83%
43% |
| Giani et al. 2021 [62] |
>65 |
144 |
56% |
5.2. Immunocompromized Patients/Pharmacological Immunosuppression
Immunosuppression is an increasing phenomenon due to medical treatments (high-dose and/or long-term steroid treatment, immunocompromising drugs, and chemotherapy), solid organ transplants, or primary immune deficiency. Owing to extended indications and early, aggressive treatments, an increasing number of patients is expected to live for many years in an immunocompromised state, which puts them at risk for severe infections. Pulmonary infections with bacteria, viruses, fungi, and even parasites are common and the leading cause for intensive care admission, often in the shade of acute respiratory failure. Infections in immunocompromised patients are associated with an increased mortality, especially if respiratory failure and the need for mechanical ventilation ensue [
63,
64]. Knowledge of the mechanism of immunosuppression in conjunction with the most likely pathogen should lead to an early and aggressive treatment of the anticipated pathogen to get the situation rapidly under control. However, if respiratory failure is severe, the question of ECMO treatment arises.
Table 4 summarizes the available studies that examined vvECMO in immunosuppressed patients. In all studies, mortality ranged up to 70%. Outcome data of specific immunosuppressed states are mostly missing.
Table 4. Evidence for the use of vvECMO in immunocompromised patients and the respective outcome.
| Study |
Disease State |
ICU Mortality |
Hospital Mortality |
Odds Ratio |
| Cawcutt et al. 2014 [65] |
HIV/AIDS |
40% |
60% |
n/a |
| Schmidt et al. 2018 [66] |
Mixed |
66% |
n/a |
n/a |
| Huprikar et al. 2019 [67] |
Acute leukemia |
n/a |
50% |
n/a |
According to available evidence, we advocate to rate immunosuppression as a relative contraindication to the initiation of vvECMO with the following consideration: if a pathogen has been identified and specific treatment is available and (about to be) started, a time-limited trial of ECMO treatment seems justified. If no pathogen has been identified or no specific treatment is available, regardless of the reasons, we advocate against the initiation of extracorporeal treatment. This statement refers only to the immunosuppressed state itself without having taken the rest of the patient’s state into consideration, which may modify the decision either way.
3.3. Time on Injurious Ventilator Settings >7 Days
Extracorporeal life support by vvECMO is currently viewed as a last-resort treatment if extended (“rescue”) conservative measures have failed to stabilize/improve respiratory status/gas exchange [
2]. However, the days spent on a ventilator prior to ECMO initiation have been shown to be an important independent factor of mortality (
Table 5). From the literature summarized in
Table 5, one can deduce that, from a time of 5 days on a ventilator, mortality starts to increase. However, only extended periods, probably 7 days or more of obviously injurious ventilation, if at all, should be considered a relative contraindication to the initiation of ECMO. Data on the effect of lung protective ventilation during the ventilator period prior to ECMO treatment are not available. In all studies so far, injurious ventilation, for example, delta pressure >15 cmH
2O and/or inspiratory pressure >35 cmH
2O, was applied in both groups! Notably, the actual ELSO guidelines state that “many centers do not consider time on ventilation a contraindication” [
68]. This is in accordance with the authors’ practice at their center.
Table 5. Evidence for the relevance of time on ventilator prior to ECMO and the respective outcome.
| Study |
Outcomes |
| Pranikoff et al. 1997 [69] |
50% mortality after 5 days on ventilator
(90% after 12 days) |
| Mols et al. 2000 [70] |
No differences between groups |
| Hemmila et al. 2004 [71] |
OR 1.20 (1.09, 1.31) (3.2 vs. 4.5 days)
(OR 5.53 if > 8 days) |
| Beiderlinden et al. 2006 [72] |
OR 1.064 (1.008, 1.123) (5.3 vs. 8.7 days) |
| Patroniti et al. 2011 [73] |
OR 1.291
(29% increase each day) |
| Schmidt et al. 2013 [74] |
p = 0.0008 between groups (3 vs. 7 days), OR 1.07 |
| Enger et al. 2014 [75] |
p = 0.013 between groups (2 vs. 5 days) |
| Mendiratta et al. 2014 [58] |
p = 0.049 between groups (1.19 vs. 1.73 days) |
| Wohlfarth et al. 2014 [76] |
p = 0.17 between groups (1 vs. 3 days) |
| Schmidt et al. 2015 [77] |
OR 1.15 (1.06, 1.26) (2 vs. 4 days) |
| Klinzing et al. 2015 [78] |
p = 0.14 between groups (1 vs. 4 days) |
| Cheng et al. 2016 [79] |
p < 0.001 between groups (1 vs. 6 days), OR 4.71 (1.98, 11.23) |
| Choi et al. 2016 [80] |
p = 0.11 between groups (4.5 vs. 4.77 days) |
| Huang et al. 2016 [81] |
p = 0.093 between groups (0.5 vs. 1.8 days) |
| Hsin et al. 2016 [82] |
p < 0.001 between groups (1 vs. 6 days) |
| Lee et al. 2016 [83] |
p = 0.114 between groups (2.3 vs. 4.2 days) |
| Serpa Neto et al. 2016 [84] |
p = 0.061 between groups (2 vs. 3 days) |
| Wu et al. 2016 [85] |
p = 0.005 between groups (2.75 vs. 6.92 days) |
| Hilder et al. 2017 [86] |
p = 0.140 between groups (1.08 vs. 1.67 days) |
| Kon et al. 2017 [87] |
OR 0.998 (0.997–0.999), p = 0.001 |
| Wu et al. 2017 [88] |
p < 0.001 between groups (1 vs. 6 days) |
| Schmidt et al. 2018 [66] |
p = 0.004 between groups (2 vs. 3 days) |
| Posluszny et al. 2020 [89] |
p = 0.028 between groups (2.33 vs. 3.25 days) |
| Giraud et al. 2021 [90] |
p = 0.01 between groups (3.79 vs. 8.67 days) |
| Supady et al. 2021 [91] |
p = 0.006 between groups (3 vs. 6 days) |
When requesting ECMO, information about the duration of (injurious) ventilation should be obtained. ECMO should be considered even in long-standing ventilation with only a short period on injurious settings or, rather, especially in these cases due to the acuity of the situation. Due to its ease of calculation, its broad availability, and close context with the mechanical properties of the lung, we actually regard the delta pressure as the most reliable marker of injurious ventilation that is widely available. Mechanical power is also a good marker for estimating the extent of injurious ventilation. However, it is undisputed that long periods of unfavorable ventilator settings in conjunction with other relative contraindications will often result in refraining from ECMO initiation.
3.4. Right-Heart Failure
No studies reporting explicitly on the effect of vvECMO on outcome in right-heart failure were available; therefore, we establish an analogy below on the basis of basic physiology.
In ARDS, right-ventricular dysfunction is common [
92,
93,
94]. It develops not only from pulmonary hypertension caused by the underlying pathophysiology itself [
95], but also from hypoxia and hypercapnia [
96,
97,
98], as well as mechanical ventilation [
99,
100,
101]. However, the effect of pulmonary hypertension in ARDS on mortality is not unequivocally established [
94,
99,
102,
103,
104]. Mechanistically, right-ventricular dysfunction might appear as an absolute contraindication for vvECMO, since the oxygenated blood from the ECMO will need to be pumped past the pulmonary circulation into the left ventricle in this setting. Recently, convincing beneficial effects on right-ventricular function due to significant decreases in mean pulmonary artery pressure after initiation of vvECMO have been reported [
105]. These effects are based on a reduction in hypoxic pulmonary vasoconstriction, as well as decreases in paCO
2 [
74,
106]. Thus, instead of starting vaECMO right away, which is associated with more complications than vvECMO [
107,
108], an approach of a trial of vvECMO and, in cases of failure, upgrading to vavECMO has been advocated [
109] and seems reasonable.
3.5. Hematologic Malignancies, Especially Bone Marrow Transplantation and Graft-Versus-Host Disease
Advances in hematologic malignancy therapy (new chemotherapeutic agents and hematologic stem-cell transplantation (HSCT)) have improved patient outcome, and it is becoming more common for these patients to require admission to the ICU due to life-threatening conditions, [
110], mainly acute respiratory failure (ARF) [
111]. Admission due to ARF is associated with poor outcomes [
112,
113,
114] and worsens if invasive mechanical ventilation is needed [
115,
116]. Nevertheless, efforts have been made in patients with hematologic malignancy to bridge ARF to recovery by use of ECMO [
76,
80,
117,
118,
119,
120,
121,
122]. All these trials conducted over a period of more than 10 years consistently demonstrated a high ICU and in-hospital mortality of more than 50% for patients treated with ECMO (
Table 6). Moreover, extremely high mortality rates of patients that developed ARF after hematologic stem-cell transplantation or graft-versus-host disease (GvHD) of 100% have been reported in four trials; two additional trials reported an in-hospital mortality of two-thirds of HSCT patients treated with ECMO.
While the prognosis for patients early after HSCT is grim, patients who acquire refractory ARDS later after HSCT may be eligible again for ECMO [
119]. Immune reconstitution is generally reacquired after 6 months, and chances of survival are probably increased by then [
123]. Therefore, the current literature suggests viewing the early phase after HSCT as an absolute contraindication to ECMO treatment. In turn, if HSCT has been completed some time ago, probably when immunosuppressive medication is tapered/immunocompetence is regained [
123], it should become a relative contraindication. Along these lines, at this time, GvHD is also very uncommon [
119].
Table 6. Evidence for the use of vvECMO in hematologic malignancies and the respective outcome.
| Study |
ICU Mortality |
Hospital Mortality |
Bone Marrow Transplant/HSCT Mortality (Hospital) |
| Gow et al. 2010 [117] |
61% |
68% |
50% |
| Wohlfarth et al. 2014 [76] |
50% |
50% |
100% |
| Kang et al. 2015 [118] |
100% |
100% |
100% |
| Choi et al. 2016 [80] |
n/a |
80.9% |
n/a |
| Wohlfarth et al. 2017 [119] |
n/a |
81% |
100% (GvHD) |
| Stecher et al. 2018 [124] |
n/a |
80% |
100% |
| Cho et al. 2019 [121] |
66% |
88% |
66.7% |
| Park et al. 2021 [122] |
n/a |
86% (OR 42.25 (9.53, 187.22)) |
85.7% (OR 64) |
3.6. SAPS II Score ≥ 60 Points
The revision of the Simplified Acute Physiology Score (SAPS II) was introduced in 1993. This score was developed and validated in a large cohort of medical and surgical patients from 137 ICUs in 12 countries with the goal of providing a relatively simple and easy-to-collect and -calculate score that would estimate the risk of death on admission to the ICU, regardless of the exact primary diagnosis [
125]. Since its publication, this score has been used extensively to compare patient populations and trials regarding their disease severity, especially in research, but also in clinical practice. Due to the timing of the development and validation of the SAPS II score in relation to the implementation of ECMO, this score has not been specifically validated in patients with ARDS/ECMO. However, in many trials involving patients on ECMO, the SAPS II score has been routinely reported as a measure of the severity, describing the investigated cohort. SAPS II has also been explicitly investigated with respect to the prediction of outcomes in patients on ECMO [
83,
91,
126,
127,
128]. All of these trials found only moderate precision in predicting mortality in patients on ECMO. One trial concluded that low mortality can be expected with a SAPS II score of less than 80 points [
126]. However, a SAPS II score of 80 points translates into a predicted mortality of more than 90%, which seems too liberal for a procedure such as ECMO. Other trials found the SAPS II score to not be helpful in outcome prediction of ECMO patients [
127]. Two articles in this Special Issue even congruently discourage the use of the SAPS II score to base the outcome prediction or the decision to initiate or refrain from extracorporeal treatment [
91,
128]. No statistically significant difference between the groups of survivors and non-survivors can be found (
p = 1.0). Nonetheless, non-survivors had a mean SAPS II score above 50, whereas survivors had a score clearly below 60 (46.89); this was confirmed in a study by Lee et al., who found a cutoff value of 58 [
83]. Therefore, we advocate a SAPS II score of 60 points or more (which translates into a predicted mortality of around 75% or more) within the last 24 h before considering extracorporeal treatment to be a relative contraindication to its initiation.
3.7. SOFA Score >12 Points (mSOFA Score >8 Points)
The Sepsis-Related/Sequential Organ Failure Assessment Score (SOFA) was created in 1996 as a result of an initiative of the European Society of Intensive Care Medicine “to quantitatively and objectively describe the degree of organ dysfunction/failure over time in groups of patients or even in individual patients” [
129]. Although, as already stated in its first description, “it is important to realize that the SOFA score is designed not to predict outcome but to describe a sequence of complications in the critically ill”, it has increasingly been used to predict mortality in various diseases/conditions [
130,
131,
132,
133,
134,
135]. All of these trials only found a fair precision in predicting mortality [
82], which obviously varies across different groups of patients. Reliable studies in vvECMO patients are largely missing.
In a mixed population of critically ill patients, a clear cutoff value of 12 points was established with respect to mortality; here, mortality jumps from around 50% to more than 80% [
129]. From our review of the literature, we can confirm this cutoff point of 12 points; the difference we found between the survivor and non-survivor groups was statistically significant (
p = 0.004). A SOFA score >12 points obviously indicates a multiorgan dysfunction syndrome (MODS) severe enough to exponentially increase mortality. Therefore, a SOFA score of greater than 12 points, regardless of whether it was measured on admission, as the highest value, or as the value at the time of indication to ECMO treatment, can strongly be advised to be a relative contraindication to its initiation.
Calculating the SOFA score in critically ill patients regularly faces inaccuracies because evaluation of the neurological status as assessed by the Glasgow Coma Scale (GCS) is very difficult in sedated patients and frequently overscores the neurological component. To account for this inaccuracy, a modified SOFA score (mSOFA) has been proposed [
136]. This modified score rates the neurological component by scoring the patient with the best assumed score without sedation (i.e., 15 if no history of neurological disorder is present).
3.8. PRESERVE Score ≥ 5 Points
In the process of searching for a specific model to predict outcome of ARDS patients and to aid in the decision to initiate ECMO, Schmidt et al. conducted a study “to identify factors associated with death by 6 months post ICU discharge for ARDS patients treated with the latest generation ECMO systems and to assess long-term survivors’ health-related quality of live (HRQL) and psycho-emotional sequelae” [
137]. In their analysis of 140 patients from three experienced ARDS/ECMO centers in France, they identified eight parameters that were independently associated with death by 6 months post ICU discharge in a multivariable analysis: age, body mass index (BMI), immunocompromised status, SAPS II, days of mechanical ventilation, no prone position before ECMO, positive end expiratory pressure (PEEP), and plateau pressure. Of these factors (with the exception that SAPS II was replaced by SOFA score), the PRESERVE (Predicting Death of Severe ARDS on vvECMO) score was derived by assigning weighted points to each parameter (age was split in three categories) and summing them to build the final score of 0 to 14 points.
From the Kaplan–Meier estimates of the derivation trial, the highest mortality rate was seen in the group of patients with a PRESERVE score ≥7 points, whereas mortality rates greater 50% can be found in patients with ≥5 points [
137]. Although different percentages have been reported, a trend that patients with a PRESERVE score ≥5 points experience high mortality rates of around and above 50% seems common [
78,
83,
91,
128,
138]. However, discrimination between survivors and non-survivors is only moderate (ROC-AUC around 0.6) for most trials. Therefore, using the PRESERVE score as the only aid for decision to initiate ECMO is generally discouraged. We propose—due to its moderate discrimination and ease of calculation—to collect this score in every patient fulfilling ECMO initiation criteria and use it as an additional source to contribute to the decision-making process.
3.9. RESP Score Worse Than −2 Points
From the same researchers that also constructed the PRESERVE score, another survival prediction score was proposed only 1 year later: the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. This score was constructed retrospectively from the ELSO database (Extracorporeal Life Support Organization) using logistic regression and bootstrapping [
139]. The score derived from this method can range from values of −22 to 15, breaking down into five risk categories (I–V). Of these, risk category IV (−2 to −5 points) already translates into a mortality rate of 67%, as has consistently been shown [
78,
83,
128,
138,
139]; in our summary of the literature, even higher/less negative scores were found in non-survivors.
The RESP score also has a moderate discrimination between survivors and non-survivors, albeit slightly better than the PRESERVE score (ROC-AUC around 0.7–0.75). Similar to PRESERVE, using this score as the only aid to decide whether to initiate ECMO or not is discouraged, but it can be used as an additional resource.
3.10. PRESET Score ≥ 6 Points
The newest of the outcome prediction scores for the initiation of ECMO therapy is the Prediction of Survival on ECMO Therapy (PRESET) score [
86]. It provides some advantages over the other risk scores mentioned before. First, it consists of only five items that are captured during daily routine (mean arterial pressure, lactate concentration, arterial pH, platelet concentration, and hospital days before ECMO). The items in each category are weighted by assigning individual points (0 to 5), which are summed to result in a final score of 0 to 15 points. The summed score allows a breakdown into three distinct risk categories that consist of 5 points each. These risk categories finally translate into a mortality prediction (category I 26% mortality, category II 68% mortality, category III 93% mortality). Interestingly, the derivation of this score only identified extrapulmonary factors that predict mortality. This fact is unexpected in light of the other prediction scores mentioned, the underlying severe lung pathology, and the known problems of ventilating these patients adequately without increasing damage to their lungs. However, this score proved to be a good predictive value in COVID-19 patients [
140], which is advantageous in light of the current pandemic. Moreover, a recent evaluation of this score revealed that it performed best amongst the other ECMO prediction scores; however, with an AUC of 0.658, the absolute performance was still only moderate. Authors of recent evaluations of ECMO prediction scores discourage the use of any of the available scores as a single decision tool [
83,
128]; however, acting with caution, the PRESET score could still be advantageous [
141].
This entry is adapted from the peer-reviewed paper 10.3390/membranes11080584