Nanocarriers are added as colloidal nanosystems loaded with therapeutic agents (anticancer agents or any macromolecules, such as proteins or genes), which allow drugs to selectively accumulate at the site of cancerous tumors. As a result of their unique nanometer range, 1–1000 nm (drug administration is preferable in the 5–200 nm range), they are used for cancer treatment. The main and most promising nanocarriers in the literature are iron oxide, gold, polymers, liposomes, micelles, fullerenes (carbon nanotubes, graphene), dendrimers, quantum dots, and nanodiamonds.
- nanocarriers
- immune system
- nanobiotechnology
- cancer
1. Introduction
Cancer is a major cause of global morbidity and mortality. It is a disease caused by a variety of factors, and its formation depends on several genetic and epigenetic aspects [1]. Malignant tumors have a specificity that affects other healthy cells in the body [2]. In order to develop more effective methods of diagnosis and treatment without harming the patient, various resources have been widely explored, and current treatment methods used for cancer control include chemotherapy, surgery, radiation, and biological therapies (immunotherapy and hormone therapy) [3][4][5].
However, these therapies have certain disadvantages and, being invasive, have side effects before and after treatment, making the patient uncomfortable. For example, the use of chemotherapeutic drugs can affect the normal and healthy growth of good cells and bring opportunities for tumor recurrence. In addition, resistance to various drugs may develop, and poor biodistribution results in a low concentration of these chemotherapeutic agents at the tumor site, which may reduce the therapeutic effect of anticancer drugs [6][7][8][9]. In this context, it is necessary to research and develop alternative beneficial and effective therapies for the drug delivery system.
Nanotechnology can increase the pharmacological properties of compounds commonly used in the treatment and diagnosis of cancer, which is why it has emerged as an innovative possibility for therapeutic intervention in cancer and in the distribution of drugs [10][11]. This can usually be achieved by different routes of administration, such as oral, nasal, transdermal, intravenous, etc. These nanocarriers can improve the effectiveness of the drug and reduce side effects. They can be encapsulated or used in combination with other drugs [12][13]. In addition, nano-scale transporters can protect drugs or any macromolecules (proteins, peptides, etc.) from degradation, reduce renal clearance, and provide sustained or controlled release kinetics, thereby increasing drug efficacy at steady-state therapeutic levels [14][15][16][17]. Their half-life in the blood improves the therapeutic index, solubility, and stability of the capsules, compared to conventional treatment methods (such as tablets, capsules, and injections) [18][19].
2. Classification of Nanocarriers
Nanocarriers can be classified into three categories based upon the materials that they are made from (A) lipid-based nanoparticles, (B) inorganic nanoparticles, and (C) polymeric nanoparticles (Figure 1).
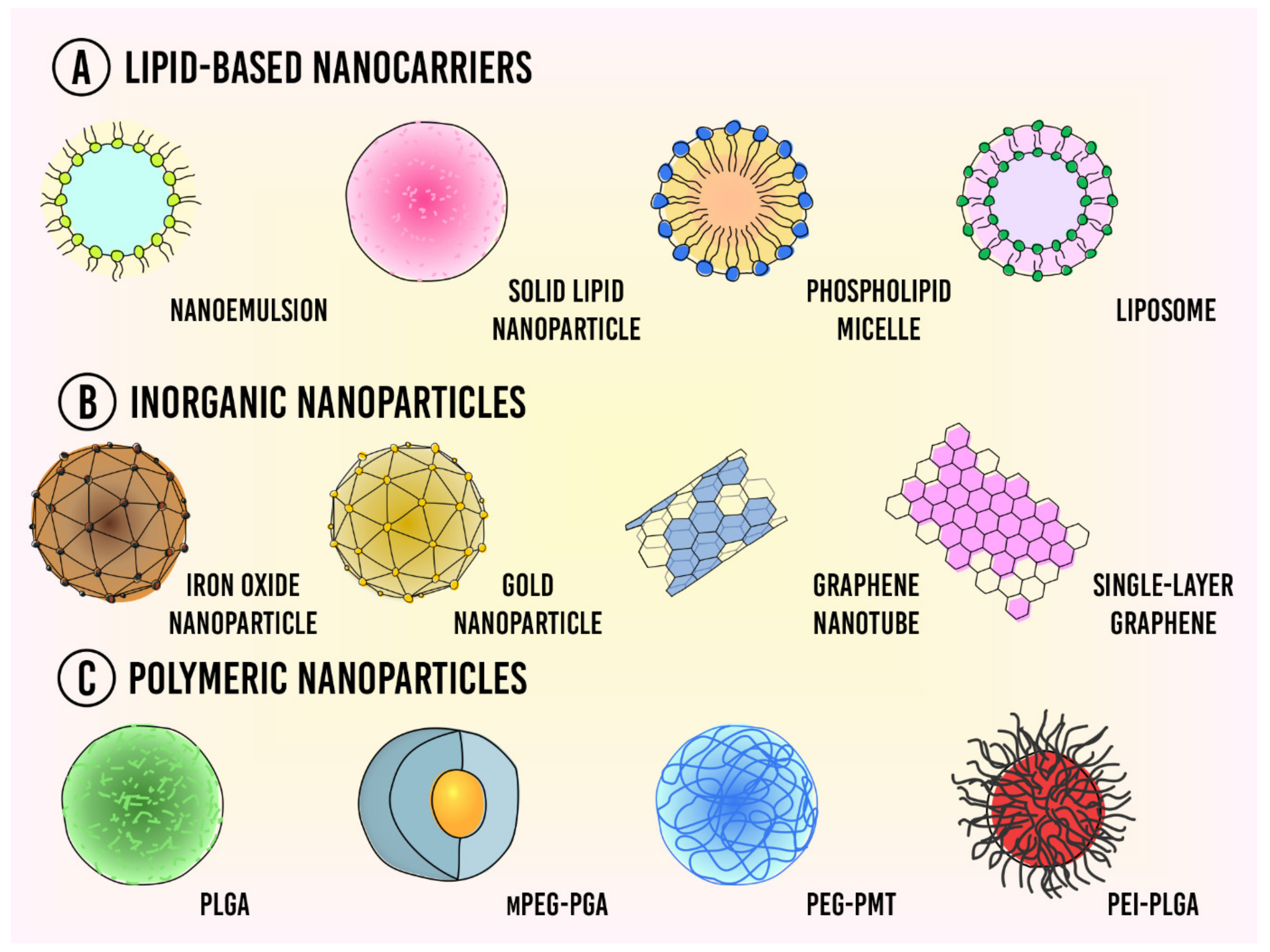 Figure 1. Types of nanocarriers used for drug delivery in cancer therapy. (A) Lipid-based nanocarriers; (B) Inorganic nanoparticles; (C) Polymeric nanoparticles.
Figure 1. Types of nanocarriers used for drug delivery in cancer therapy. (A) Lipid-based nanocarriers; (B) Inorganic nanoparticles; (C) Polymeric nanoparticles.
2.1. Lipid-Based Nanocarriers
2.2. Polymeric Nanocarriers
2.3. Inorganic Nanoparticles
3. Conclusions and Future Prospects
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13081167
References
- Vanza, J.D.; Patel, R.B.; Patel, M.R. Nanocarrier centered therapeutic approaches: Recent developments with insight towards the future in the management of lung cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 102070.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143.
- Shi, Y.; Lammers, T. Combining Nanomedicine and Immunotherapy. Accounts Chem. Res. 2019, 52, 1543–1554.
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221.
- Lv, Y.; Tao, L.; Bligh, S.A.; Yang, H.; Pan, Q.; Zhu, L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater. Sci. Eng. C 2016, 59, 652–660.
- Guido, C.; Maiorano, G.; Cortese, B.; D’Amone, S.; Palamà, I.E. Biomimetic Nanocarriers for Cancer Target Therapy. Bioengineering 2020, 7, 111.
- Bahrami, B.; Farsangi, M.H.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83.
- Ehsanimehr, S.; Moghadam, P.N.; Dehaen, W.; Shafiei-Irannejad, V. Synthesis of pH-sensitive nanocarriers based on polyacrylamide grafted nanocrystalline cellulose for targeted drug delivery to folate receptor in breast cancer cells. Eur. Polym. J. 2021, 150, 110398.
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70.
- Fang, X.; Cao, J.; Shen, A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662.
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 101–109.
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1–15.
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36.
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205.
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264.
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323.
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755.
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 72–91.
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating Nanotechnology into Cancer Care. ACS Nano 2019, 13, 7370–7376.
- Bangham, A.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–IN10.
- Forssen, E.A.; Ross, M.E. Daunoxome® Treatment of Solid Tumors: Preclinical and Clinical Investigations. J. Liposome Res. 1994, 4, 481–512.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Kulkarni, J.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157.
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966.
- Sapra, P.T.A.T.M.A.P.; Tyagi, P.; Allen, T.M. Ligand-Targeted Liposomes for Cancer Treatment. Curr. Drug Deliv. 2005, 2, 369–381.
- Shamant, B.S.; Moin, A.; Gowda, D.V.; Rashmi, R.; Hiremath, R. Lipid based drug delivery systems in arthritis and allied conditions. World J. Pharm. Sci. 2016, 4, 61–68.
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 1–10.
- Ansari, M.T.; Ramlan, T.A.; Jamaluddin, N.N.; Zamri, N.; Salfi, R.; Khan, A.; Sami, F.; Majeed, S.; Hasnain, M.S. Lipid-based Nanocarriers for Cancer and Tumor Treatment. Curr. Pharm. Des. 2020, 26, 4272–4276.
- Beck-Broichsitter, M.; Merkel, O.; Kissel, T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J. Control. Release 2012, 161, 214–224.
- Ahuja, R.; Panwar, N.; Meena, J.; Singh, M.; Sarkar, D.P.; Panda, A.K. Natural products and polymeric nanocarriers for cancer treatment: A review. Environ. Chem. Lett. 2020, 18, 2021–2030.
- Vijayan, V.M.; Muthu, J. Polymeric nanocarriers for cancer theranostics. Polym. Adv. Technol. 2017, 28, 1572–1582.
- Sun, Q.; Zhu, Y.; Du, J. Recent progress on charge-reversal polymeric nanocarriers for cancer treatments. Biomed. Mater. 2021, 16, 042010.
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298.
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265.
- Desai, P.; Date, A.; Patravale, V.B. Overcoming poor oral bioavailability using nanoparticle formulations—Opportunities and limitations. Drug Discov. Today Technol. 2012, 9, e87–e95.
- Gao, F.; Xiong, Z. Reactive Oxygen Species Responsive Polymers for Drug Delivery Systems. Front. Chem. 2021, 9, 66.
- Dhas, N.; Ige, P.P.; Kudarha, R. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer. Powder Technol. 2015, 283, 234–245.
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359.
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803.
- Paul, W.; Sharma, C.P. Inorganic nanoparticles for targeted drug delivery. Biointegration Med. Implant. Mater. 2020, 334–373.
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827.
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003.
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51.
- Behbudi, G. Mini review of Graphene Oxide for medical detection and applications. Adv. Appl. NanoBio-Technol. 2020, 1, 63–66.
- Alphandéry, E. Iron oxide nanoparticles for therapeutic Applications. Drug Discov. Today 2019, 25, 141–149.
- Li, M.; Kim, H.S.; Tian, L.; Yu, M.K.; Jon, S.; Moon, W.K. Comparison of Two Ultrasmall Superparamagnetic Iron Oxides on Cytotoxicity and MR Imaging of Tumors. Theranostics 2012, 2, 76–85.
- Duncan, R.; Gaspar, R. Nanomedicine (s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141.
- Carneiro, M.L.B.; Peixoto, R.C.; Joanitti, G.A.; Oliveira, R.G.; Telles, L.A.; Miranda-Vilela, A.L.; Bocca, A.L.; Vianna, L.M.; Da Silva, I.C.; De Souza, A.R.; et al. Antitumor effect and toxicity of free rhodium (II) citrate and rhodium (II) citrate-loaded maghemite nanoparticles in mice bearing breast cancer. J. Nanobiotechnol. 2013, 11, 4.
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596.
- Nagesh, P.K.B.; Johnson, N.R.; Boya, V.K.; Chowdhury, P.; Othman, S.F.; Khalilzad-Sharghi, V.; Hafeez, B.B.; Ganju, A.; Khan, S.; Behrman, S.W.; et al. PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf. B Biointerfaces 2016, 144, 8–20.
- Estelrich, J.; Brusquets, M.A. Iron oxide nanoparticles in photothermal therapy. Molecules 2016, 408, 1567.
- Patel, A.; Sant, S. Hypoxic tumor microenvironment: Opportunities to develop targeted therapies. Biotechnol. Adv. 2016, 34, 803–812.
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S. Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles for Doxorubicin Delivery: Synthesis and Anticancer Effect Against Human Ovarian Cancer Cells. Chem. Biol. Drug Des. 2013, 82, 296–306.
- Lee, C.-S.; Kim, H.; Yu, J.; Yu, S.H.; Ban, S.; Oh, S.; Jeong, D.; Im, J.; Baek, M.J.; Kim, T.H. Doxorubicin-loaded oligonucleotide conjugated gold nanoparticles: A promising in vivo drug delivery system for colorectal cancer therapy. Eur. J. Med. Chem. 2017, 142, 416–423.
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824.
- Dos Santos, M.S.C.; Gouvêa, A.L.; de Moura, L.D.; Paterno, L.G.; de Souza, P.E.N.; Bastos, A.P.; Damaceno, E.A.M.; Veiga-Souza, F.H.; Azevedo, R.B.; Báo, S.N. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 1–17.
- Deng, X.; Liang, H.; Yang, W.; Shao, Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111913.
