β-adrenergic receptor stimulation (β-ARS) is a physiological mechanism that regulates cardiovascular function under stress conditions or physical exercise, producing a positive inotropic (enhanced contraction), lusitropic (faster relaxation), and chronotropic (increased heart rate) effect.
- β-adrenergic receptor stimulation
- mathematical modelling
- cardiac electrophysiology
- cardiomyocyte
1. Introduction
β-adrenergic receptor stimulation (β-ARS) is a physiological response mechanism that plays a fundamental role in the regulation of cardiomyocyte activity, producing a positive inotropic (enhanced contraction), lusitropic (faster relaxation), and chronotropic (increased heart rate) effect. Such a multifactorial response is triggered via the activation of the β-adrenergic receptors by the sympathetic nervous system, under either stress conditions or physical exercise, and is also known as the “fight-or-flight” response.
β-adrenergic receptors were first described by Lands et al. in the late 1960s [1][2]. They are situated on the cardiomyocyte membrane and react to different neurotransmitters (norepinephrine, epinephrine) and drugs (isoprenaline). When binding with the adrenergic receptor takes place, it starts a reaction cascade where different cellular substrates become phosphorylated, affecting their individual roles in the overall excitation–contraction coupling. As a result, under healthy physiological conditions, the cardiac action potential shortens, while the intracellular calcium transient exhibits an increased amplitude and a faster decay rate as the main manifestations of β-ARS at the cellular level [3][4]. However, the large number of components involved in the β-adrenergic cascade, and the complexity of these subcellular processes and interactions, make β-ARS signalling highly sensitive to cellular changes and to pathological perturbations. As a result, β-ARS plays a main role in a considerable number of heart diseases [5], and it is well established as an important contributor to cardiomyocyte arrhythmogenicity [6][7][8].
In particular, the β-ARS response has direct effects on the ion channels and pumps of the cell membrane (and, therefore, on intracellular ionic concentrations) regulating calcium intake, intracellular calcium handling, calcium extrusion, and cellular repolarisation. Impairments in the balance between these carefully orchestrated processes can affect heart function and render its constituent cardiomyocytes susceptible to proarrhythmic events, such as early and delayed afterdepolarisations (EADs and DADs, respectively). Such afterdepolarisations under β-ARS are common proarrhythmic manifestations in isolated cardiomyocytes from patients of different pathologies, especially those characterised by action potential and calcium transient abnormalities (such as hypertrophic cardiomyopathy [9], long QT syndrome [10][11], or catecholaminergic polymorphic ventricular tachycardia [12]). An overstimulated β-ARS response is also one of the main mechanisms of cardiac hypertrophy, coronary artery disease, or stroke events [13]. The overexpression of the β-adrenergic response has also been linked to the onset of cardiac hypertrophy or the generation of fibrotic tissue [14][15]. The appearance of these structural changes can lead to the creation of re-entry pathways in myocardial tissue, which also contribute to the generation of self-sustained arrhythmias. Induced arrhythmias are also a common manifestation in heart failure. In chronic heart failure, cardiac remodelling at the structural level can affect the pathways involved in the β-ARS response [16]. As a result, the inotropic response of cardiomyocytes to β-ARS is reduced [17][18], while the propensity to arrhythmogenic events increases. β-adrenergic response is also affected by ageing, and an age-dependent impairment between β-ARS and cardiac function has been demonstrated in both healthy and failing hearts [19][20]. β-ARS is altered as well in severe congenital heart disease patients [21]. Finally, recent studies also suggest a hyperactivation of the positive response of β-ARS patients with coronavirus disease 2019 (COVID-19), potentially leading to life-threatening arrhythmic events [22][23].
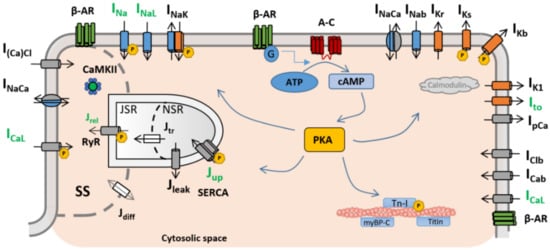
Refined knowledge of the role that each cellular component has within the β-ARS cascade, and of the consequences that may arise from disturbing its normal functioning, can therefore lead to a better understanding of different pathologies, as well as to the development of new pharmacological targets for their treatment. In these cases, mathematical modelling and simulation studies of β-ARS can be useful tools for investigating the mechanisms mediating arrhythmic events, assessing their multiscale consequences from the subcellular up to the organ levels, and designing effective treatments [24]. Here, we review the main roles of β-ARS in cellular cardiac electrophysiology, placing our emphasis on the description of the existing mathematical frameworks available for its representation and how insights obtained through experimental approaches have been integrated into these mathematical formulations.
2. Mathematical Models of β-ARS
| Model (Year) | Species | β-ARS Isoform |
Signalling | Substrates | Main Model Advances |
|---|---|---|---|---|---|
| Zeng and Rudy [35] (1995) |
Guinea Pig | Generic | None | ICaL; IK; PLB; INaK; INa | Simulation of the isoproterenol effect by increasing conductances and parameter shift |
| Saucerman et al. [36] (2003) |
Rat | β1 | cAMP; PKA | ICaL; PLB | Dynamic target phosphorylation integrated with cell signalling |
| Greenstein et al. [37] (2004) |
Dog | Generic | None | ICaL; PLB; IKr; IKs | Introduction of a binary population-based approach for target phosphorylation |
| Iancu et al. * [38] (2007) |
Guinea Pig | β1 | cAMP | N/A | Cellular signalling compartmentation |
| Soltis & Saucerman [29] (2010) | Rabbit | β1 | cAMP; PKA | ICaL; IKs; PLB; RyR; TnI; ICFTR | Integration with dynamic CaMKII regulation |
| Hiejman et al. [39] (2011) |
Dog | β1, β2 | cAMP; PKA | ICaL; IKs; IKur; PLB; INaK; INa; RyR; TnI | Two different β isoforms; population-based approach with four different populations |
| Bondarenko [40] (2014) |
Mouse | β1 | cAMP; PKA | ICaL; INa; INaK; RyR; IKur; Ito; IK1; PLB; TnI | Compartmentalised mouse model with new L-type calcium channel subpopulations |
| Khalilimeybodi et al. * [41] (2018) | Mouse | β1, β2 | cAMP; PKA; GSK3β; ERK | N/A | Addition of new molecular signalling pathways (GSK3β and ERK) |
This entry is adapted from the peer-reviewed paper 10.3390/math9151785
References
- Lands, A.M.; Arnold, A.; McAuliff, J.P.; Luduena, F.P.; Brown, T.G. Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967, 214, 597–598.
- Lands, A.M.; Luduena, F.P.; Buzzo, H.J. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967, 6, 2241–2249.
- Coppini, R.; Ferrantini, C.; Yao, L.; Fan, P.; Del Lungo, M.; Stillitano, F.; Sartiani, L.; Tosi, B.; Suffredini, S.; Tesi, C.; et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 2013, 127, 575–584.
- Guo, G.R.; Chen, L.; Rao, M.; Chen, K.; Song, J.P.; Hu, S.S. A modified method for isolation of human cardiomyocytes to model cardiac diseases. J. Transl. Med. 2018, 16, 288.
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274.
- Pogwizd, S.M.; Schlotthauer, K.; Li, L.; Yuan, W.; Bers, D.M. Arrhythmogenesis and Contractile Dysfunction in Heart Failure. Circ. Res. 2001, 88, 1159–1167.
- Desantiago, J.; Ai, X.; Islam, M.; Acuna, G.; Ziolo, M.T.; Bers, D.M.; Pogwizd, S.M. Arrhythmogenic effects of β2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ. Res. 2008, 102, 1389–1397.
- Myles, R.C.; Wang, L.; Kang, C.; Bers, D.M.; Ripplinger, C.M. Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ. Res. 2012, 110, 1454–1464.
- Ferrantini, C.; Pioner, J.M.; Mazzoni, L.; Gentile, F.; Tosi, B.; Rossi, A.; Belardinelli, L.; Tesi, C.; Palandri, C.; Matucci, R.; et al. Late sodium current inhibitors to treat exercise-induced obstruction in hypertrophic cardiomyopathy: An in vitro study in human myocardium. Br. J. Pharmacol. 2018, 175, 2635–2652.
- Goldenberg, I.; Thottathil, P.; Lopes, C.M.; Moss, A.J.; McNitt, S.; Jin, O.U.; Robinson, J.L.; Zareba, W.; Ackerman, M.J.; Kaufman, E.S.; et al. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm 2012, 9, 49–56.
- Uchi, J.; Rice, J.J.; Ruwald, M.H.; Parks, X.X.; Ronzier, E.; Moss, A.J.; Zareba, W.; Lopes, C.M. Impaired IKs channel activation by Ca2+-dependent PKC shows correlation with emotion/arousal-triggered events in LQT1. J. Mol. Cell. Cardiol. 2015, 79, 203–211.
- Danielsen, T.K.; Manotheepan, R.; Sadredini, M.; Leren, I.S.; Edwards, A.G.; Vincent, K.P.; Lehnart, S.E.; Sejersted, O.M.; Sjaastad, I.; Haugaa, K.H.; et al. Arrhythmia initiation in catecholaminergic polymorphic ventricular tachycardia type 1 depends on both heart rate and sympathetic stimulation. PLoS ONE 2018, 13, e0207100.
- Shin, E.; Ko, K.S.; Rhee, B.D.; Han, J.; Kim, N. Different effects of prolonged β-adrenergic stimulation on heart and cerebral artery. Integr. Med. Res. 2014, 3, 204–210.
- Engelhardt, S.; Hein, L.; Wiesmann, F.; Lohse, M.J. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7059–7064.
- Ostrom, R.S.; Naugle, J.E.; Hase, M.; Gregorian, C.; Swaney, J.S.; Insel, P.A.; Brunton, L.L.; Meszaros, J.G. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin: Gq-Ga cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003, 278, 24461–24468.
- Johnson, D.M.; Antoons, G. Arrhythmogenic Mechanisms in Heart Failure: Linking β-Adrenergic Stimulation, Stretch, and Calcium. Front. Physiol. 2018, 9, 1453.
- Beuckelmann, D.J.; Erdmann, E. Ca2+-currents and intracellular [Ca2+]i-transients in single ventricular myocytes isolated from terminally failing human myocardium. In Cellular and Molecular Alterations in the Failing Human Heart; Steinkopff: Heidelberg, Germany, 1992; pp. 235–243.
- Harding, S.E.; Jones, S.M.; O’Gara, P.; Vescovo, G.; Poole-Wilson, P.A. Reduced β-agonist sensitivity in single atrial cells from failing human hearts. Am. J. Physiol. Heart Circ. Physiol. 1990, 259.
- Leosco, D.; Rengo, G.; Iaccarino, G.; Filippelli, A.; Lymperopoulos, A.; Zincarelli, C.; Fortunato, F.; Golino, L.; Marchese, M.; Esposito, G.; et al. Exercise training and β-blocker treatment ameliorate age-dependent impairment of β-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1596–1603.
- Lucia, C.D.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904.
- Kozlik-Feldmann, R.; Kramer, H.H.; Wicht, H.; Feldmann, R.; Netz, H.; Reinhardt, D. Distribution of Myocardial β-Adrenoceptor Subtypes and Coupling to the Adenylate Cyclase in Children With Congenital Heart Disease and Implications for Treatment. J. Clin. Pharmacol. 1993, 33, 588–595.
- Lazzerini, P.E.; Boutjdir, M.; Capecchi, P.L. COVID-19, Arrhythmic Risk, and Inflammation. Circulation 2020, 142, 7–9.
- Sutanto, H.; Heijman, J. Beta-Adrenergic Receptor Stimulation Modulates the Cellular Proarrhythmic Effects of Chloroquine and Azithromycin. Front. Physiol. 2020, 11, 587709.
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The “Digital Twin” to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564.
- Woo, A.Y.H.; Xiao, R.P. β-Adrenergic receptor subtype signaling in heart: From bench to bedside. Acta Pharmacol. Sin. 2012, 33, 335–341.
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49.
- Volders, P.G.A.; Stengl, M.; Van Opstal, J.M.; Gerlach, U.; Spätjens, R.L.H.M.G.; Beekman, J.D.M.; Sipido, K.R.; Vos, M.A. Probing the contribution of IKs to canine ventricular repolarization: Key role for β-adrenergic receptor stimulation. Circulation 2003, 107, 2753–2760.
- Baba, S.; Dun, W.; Boyden, P.A. Can PKA activators rescue Na+ channel function in epicardial border zone cells that survive in the infarcted canine heart? Cardiovasc. Res. 2004, 64, 260–267.
- Soltis, A.R.; Saucerman, J.J. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. Biophys. J. 2010, 99, 2038–2047.
- Negroni, J.A.; Morotti, S.; Lascano, E.C.; Gomes, A.V.; Grandi, E.; Puglisi, J.L.; Bers, D.M. Β-Adrenergic Effects on Cardiac Myofilaments and Contraction in an Integrated Rabbit Ventricular Myocyte Model. J. Mol. Cell. Cardiol. 2015, 81, 162–175.
- Despa, S.; Bossuyt, J.; Han, F.; Ginsburg, K.S.; Jia, L.G.; Kutchai, H.; Tucker, A.L.; Bers, D.M. Phospholemman-phosphorylation mediates the β-adrenergic effects on Na/K pump function in cardiac myocytes. Circ. Res. 2005, 97, 252–259.
- Ginsburg, K.S.; Bers, D.M. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J. Physiol. 2004, 556, 463–480.
- Stelzer, J.E.; Patel, J.R.; Walker, J.W.; Moss, R.L. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ. Res. 2007, 101, 503–511.
- Krüger, M.; Linke, W.A. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J. Muscle Res. Cell Motil. 2006, 27, 435–444.
- Zeng, J.; Rudy, Y. Early afterdepolarizations in cardiac myocytes: Mechanism and rate dependence. Biophys. J. 1995, 68, 949–964.
- Saucerman, J.J.; Brunton, L.L.; Michailova, A.P.; McCulloch, A.D. Modeling β-Adrenergic Control of Cardiac Myocyte Contractility in Silico. J. Biol. Chem. 2003, 278, 47997–48003.
- Greenstein, J.L.; Tanskanen, A.J.; Winslow, R.L. Modeling the actions of β-adrenergic signaling on excitation- contraction coupling processes. Ann. N. Y. Acad. Sci. 2004, 1015, 16–27.
- Iancu, R.V.; Jones, S.W.; Harvey, R.D. Compartmentation of cAMP signaling in cardiac myocytes: A computational study. Biophys. J. 2007, 92, 3317–3331.
- Heijman, J.; Volders, P.G.A.; Westra, R.L.; Rudy, Y. Local control of β-adrenergic stimulation: Effects on ventricular myocyte electrophysiology and Ca2+-transient. J. Mol. Cell. Cardiol. 2011, 50, 863–871.
- Bondarenko, V.E. A Compartmentalized Mathematical Model of the β1-Adrenergic Signaling System in Mouse Ventricular Myocytes. PLoS ONE 2014, 9, e89113.
- Khalilimeybodi, A.; Daneshmehr, A.; Sharif-Kashani, B. Investigating β-adrenergic-induced cardiac hypertrophy through computational approach: Classical and non-classical pathways. J. Physiol. Sci. 2018, 68, 503–520.
- Tomek, J.; Bueno-Orovio, A.; Passini, E.; Zhou, X.; Minchole, A.; Britton, O.; Bartolucci, C.; Severi, S.; Shrier, A.; Virag, L.; et al. Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. eLife 2019, 8, e48890.
- Tanskanen, A.J.; Greenstein, J.L.; O’Rourke, B.; Winslow, R.L. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys. J. 2005, 88, 85–95.
- Saucerman, J.J.; McCulloch, A.D. Mechanistic systems models of cell signaling networks: A case study of myocyte adrenergic regulation. Prog. Biophys. Mol. Biol. 2004, 85, 261–278.
- Zaccolo, M.; Pozzan, T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 2002, 295, 1711–1715.
- Kuznetsov, V.; Pak, E.; Robinson, R.B.; Steinberg, S.F. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ. Res. 1995, 76, 40–52.
- Vittone, L.; Mundiña-Weilenmann, C.; Said, M.; Mattiazzi, A. Mechanisms involved in the acidosis enhancement of the isoproterenol-induced phosphorylation of phospholamban in the intact heart. J. Biol. Chem. 1998, 273, 9804–9811.
- Xiao, R.P.; Lakatta, E.G. β1-Adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ. Res. 1993, 73, 286–300.
- Yang, J.H.; Saucerman, J.J. Phospholemman is a negative feed-forward regulator of Ca2+ in β-adrenergic signaling, accelerating β-adrenergic inotropy. J. Mol. Cell. Cardiol. 2012, 52, 1048–1055.
- Meyer, E.E.; Clancy, C.E.; Lewis, T.J. Dynamics of adrenergic signaling in cardiac myocytes and implications for pharmacological treatment. J. Theor. Biol. 2021, 519, 110619.
- Warrier, S.; Belevych, A.E.; Ruse, M.; Eckert, R.L.; Zaccolo, M.; Pozzan, T.; Harvey, R.D. β-adrenergic- and muscarinic receptor-induced changes in cAMP activity in adult cardiac myocytes detected with FRET-based biosensor. Am. J. Physiol. Cell Physiol. 2005, 289, 455–461.
- Hohl, C.M.; Li, Q. Compartmentation of cAMP in adult canine ventricular myocytes: Relation to single-cell free Ca2+ transients. Circ. Res. 1991, 69, 1369–1379.
- Nagykaldi, Z.; Kem, D.; Lazzara, R.; Szabo, B. Canine ventricular myocyte β2-adrenoceptors are not functionally coupled to L-type calcium current. J. Cardiovasc. Electrophysiol. 1999, 10, 1240–1251.
- Johnson, D.M.; Heijman, J.; Pollard, C.E.; Valentin, J.P.; Crijns, H.J.G.M.; Abi-Gerges, N.; Volders, P.G.A. IKs restricts excessive beat-to-beat variability of repolarization during beta-adrenergic receptor stimulation. J. Mol. Cell. Cardiol. 2010, 48, 122–130.
- O’Hara, T.; Rudy, Y. Arrhythmia formation in subclinical (“silent”) long QT syndrome requires multiple insults: Quantitative mechanistic study using the KCNQ1 mutation Q357R as example. Heart Rhythm 2012, 9, 275–282.
- Gong, J.Q.X.; Susilo, M.E.; Sher, A.; Musante, C.J.; Sobie, E.A. Quantitative analysis of variability in an integrated model of human ventricular electrophysiology and β-adrenergic signaling. J. Mol. Cell. Cardiol. 2020, 143, 96–106.
