Quorum sensing (QS) is the mechanism by which the microbial colonies in a biofilm modulate and intercept communication without direct interaction. Hence, the eradication of biofilms through hindering this communication will lead to the successful management of drug resistance and may be a novel target for antimicrobial chemotherapy. Chitosan shows microbicidal activities by acting electrostatically with its positively charged amino groups, which interact with anionic moieties on microbial species, causing enhanced membrane permeability and eventual cell death. Therefore, nanoparticles (NPs) prepared with chitosan possess a positive surface charge and mucoadhesive properties that can adhere to microbial mucus membranes and release their drug load in a constant release manner. As the success in therapeutics depends on the targeted delivery of drugs, chitosan nanomaterial, which displays low toxicity, can be safely used for eradicating a biofilm through attenuating the quorum sensing (QS).
1. Introduction
The last decade has seen a marked increase in the development of multi-drug-resistant pathogenic organisms that have brought about significant threats for the health sector. Numerous alternative approaches are being taken to check the pathogenesis of these antibiotic-resistant microbes and strategies are being adopted to minimize their virulence [
1,
2]. The chronic-infection-causing recalcitrant microbes usually reside in the protective shield of their biofilm, which is actually a syntrophic association of microbes. Hence, exploration of the natural ways for biofilm eradication and innovations for biotechnological approaches to enhance their antibiofilm activity becomes a new and booming stream of research.
Both microbes alone and the biofilm formed by them attach themselves to specific surfaces. The biofilm-associated cells are especially capable of forming an extracellular polymeric substance matrix (EPS), which can maintain decreased growth rates and allow for up- and down regulation of some specific genes [
3,
4]. The EPS matrix possesses a definite construction pattern and creates an optimal condition that allows the microbes to exchange genetic contents between the cells [
5]. Moreover, the biofilm-forming cells undergo cell-to-cell communication via the process of quorum sensing (QS), by which they control the expression of genetic components in response to continuous changes in the density of the cell population [
6]. QS is accomplished by various types of extracellular communication materials called autoinducers (AIs) [
7], which are the chemical signaling molecules that are synthesized and released by these cells [
8].
Since QS plays a key role in bacterial infection and bacterial survival, eradication of the biofilm through the denaturation of the AI molecules will ensure the prevention of biofilm-associated infection. Several novel antibiofilm agents were developed for interfering with the QS cascade and thereby inhibiting the formation of biofilms [
9,
10].
However, such interruption in cellular communication can be done via the mechanism of quorum quenching (QQ), which involves the process of disrupting the QS cascade [
7]. The molecular mechanism of QQ includes the cleavage of QS signals, competitive inhibition, and acting against the major targets of QS, thereby bringing about hindrance in the maturation of biofilm.
Present-day nanomaterials are largely used as alternate therapeutics due to their large surface-area-to-volume ratio and extensive reactivity, resulting in the development of the new field of “nanomedicines” [
11,
12,
13]. The enhancement in the development of antimicrobial resistance has resulted in researchers thinking about ways to provide alternate therapeutics [
14]. A fascinating thing about nanomaterials is that their efficacies are largely dependent on the shape and size of the nanostructural contents of the nanomaterials and these properties can usually be distinguished well from the bulk traditional material, which possesses the appearance of a continuous material [
15]. This is why these nanomaterials create huge interest regarding their applications in different types of research and development fields related to biotechnology, biology, chemistry, biophysics, and many others [
16].
2. Quorum Sensing in Biofilm-Associated Microbes
QS, being the key event behind biofilm formation, is the main target for blocking to achieve an antibiofilm effect. Apart from biofilm formation, QS regulates multiple processes that involve sporulation, bioluminescence, the production of various types of virulence factors, antibiotic biosynthesis, and the formation of biofilms [
31,
32]. The mechanism of QS in Gram-negative bacteria (
Table 1) takes place via LuxI/LuxR type systems, which play an important role in the production of AIs, the signalling molecules [
33].
Table 1. Quorum-sensing (QS) systems of selected Gram-negative bacteria.
|
SL No.
|
Bacterial Organism Name
|
Quorum-Sensing Molecules
|
Genes
|
Receptors
|
References
|
|
1.
|
Chromobacterium violaceum
|
C12-HSL
|
N.A.
|
N.A.
|
[34]
|
|
N.A.
|
N.A.
|
SdiA
|
[35]
|
|
AI-2
|
LuxS
|
LsrB
|
[16,36]
|
|
2.
|
Pseudomonas aeruginosa
|
C4-HSL
|
RhlI
|
RhlR
|
[37]
|
|
3-oxo-C12-HSL
|
LasI
|
LasR
|
[38,39]
|
|
3-oxo-C12-HSL
|
NA
|
QscR
|
[3,40]
|
|
PQS, HHQ
|
PqsABCD, PqsH
|
PqsR
|
[41]
|
|
3.
|
Staphylococcus aureus
|
3-hydroxy-C4-HSL
|
LuxM
|
LuxN
|
[3,40]
|
|
AI-2
|
LuxS
|
LuxP
|
[42]
|
|
CAI-1
|
CqsA
|
CqsS
|
[43]
|
|
4.
|
Acinetobacter baumannii
|
3-hydroxy-C12-HSL
|
AbaI
|
AbaR
|
[44]
|
|
5.
|
Escherichia coli
|
3-oxo-C8-HSL
|
N.A.
|
SdiA
|
[27,35]
|
|
AI-2
|
LuxS
|
LsrB
|
[37,45,46]
|
|
AI-3/Epinephrine/Norepinephrine
|
N.A.
|
QseC
|
[47]
|
|
6.
|
Klebsiella pneumoniae
|
C8-HSL
|
N.A.
|
N.A.
|
[15,36]
|
|
C12-HSL
|
N.A.
|
N.A.
|
[27]
|
|
AI-2
|
LuxS
|
LsrB
|
[48,49]
|
3. Chitosan Nanoparticles
A biofilm matrix acting as a scaffold provides a protective covering for sessile bacteria, making them drug resistant [
50]. Hence, a more effective drug delivery system needs to be applied that can target both the biofilm matrix and the embedded sessile bacterial cells. Chitosan and its derivatives, with their acclaimed biofilm inhibiting property, may be used but in a more precise manner to halt the quorum sensing.
Nanoparticles, with atomic dimensions of 10Å to 100Å [
51] were shown to be quite effective for drug delivery. Despite a few drawbacks, including poor absorption and dissolution rate with reduced bioavailability, using nanoparticles is a much safer method, as these microscopic particles act as nanocarriers, encasing high drug payloads and provide more targeted action with a controlled release.
Chitin, a natural polymer of β-(1,4)-N-acetyl-D-glucosamine, turns into chitosan, a polysaccharide composed of N-acetylglucosamine and D-glucosamine units [
52], upon deacetylation in the presence of an alkali. Due to its cationic nature, biodegradability, compatibility, and nontoxicity, chitosan is used extensively by nano-biomedical researchers [
53,
54,
55] for the delivery and controlled release of biomolecules, such as proteins, peptides, enzymes, genes, vaccines, and small drug molecules [
56] via various delivery routes, including oral, buccal, vaginal, and pulmonary. Chitosan NPs are also used as vaccine adjuvants due to the mucoadhesive properties of chit, which can stimulate the cells of the immune system [
57]. Some of the important properties of chitosan that have led to its wide range of applications in various fields (such as NPs) include mucoadhesion (as shown by trimethyl chitosan and carboxymethyl chitosan) [
58]; controlled drug release, which enhances its effectiveness for drug delivery [
59]; permeation enhancement, as shown by trimethyl chitosan [
60]; antibacterial activity; no cytotoxicity; biocompatibility; and biodegradability. These properties are incredibly advantageous for the advancement of biocompatible and biodegradable medication conveyance frameworks [
61,
62].
Since its first emergence in the mid-1990s, the chitosan nanoparticle (ChNP) has been used for drug delivery [
63]. The property that is responsible for the success of ChNPs in drug delivery is its ability to bind with negatively charged anions to form beads. However, beads larger than approximately 2mm generally hinder this process [
64]. The discovery of the ChNPs involves various ‘bottom-up’ or ‘top-down’ approaches, or a synergistic combination of both techniques. However, among the regular ‘bottom-up’ methods, the most popular ones are ionotropic gelation and the polyelectrolyte complex method [
65] due to their straightforwardness and non-requirement of high shear power and natural solvents [
66], unlike the ‘top-down’ methods of milling, ultrasonication, and high-pressure homogenization [
62,
67].Irrespective of the methodology adopted for their preparation, the ChNPs are regularly used for drug delivery to combat several diseases with appreciable efficacy. Although the precise mode of antimicrobial action is not determined completely, it was proposed that the molecular structure of chitosan is imperative for its antimicrobial activities. The antibacterial potential of chitosan is strongly influenced by several factors, such as its type, degree of polymerization, and physicochemical properties.
4. Inhibition of Biofilm Formation Using Functionalized Chitosan Nanoparticles
However, in order to target biofilm-associated chronic infections, medical devices, and food industries [
110], the ChNPs must have the ability to block quorum sensing. It was revealed from various experimental observations that the positively charged ChNPs are usually loaded in Oxa or oxacillin and ChNP–DNase–Oxa or Deoxyribonuclease I [
111]. The anti-biofilm activity is generally studied against the biofilm network formed by nosocomial bacterial species, such as
Staphylococcus aureus and
Pseudomonas aeruginosa. Biofilm structuring on silicone surfaces was checked and researched with the help of SEM or scanning electron microscopy [
112]. Confocal laser scanning microscopy (CLSM) was used for looking upon alive or dead microorganisms inside the biofilm matrix, which revealed that ChNP–DNase–Oxa had a higher level of anti-biofilm activity than the Oxa-mixed nanoparticles, which is present without the ChNP–Oxa or the DNase and the summation of Oxa and DNase, which involves free Oxa [
41,
113]. Both the formation of new biofilms and the eradication of mature biofilms in vitro could be achieved with the help of the ChNP–DNase–Oxa. Actually, through the denaturation of eDNA, ChNP–DNase–Oxa can damage the biofilm matrix, decrease the width of the biofilm, and the number of viable cells on silicone. Back-to-back treating with the help of ChNP–DNase–Oxa over two days was seen to give a shocking and successful result of almost a 99% decrease in the biofilm [
114]. Moreover, ChNP–DNase–Oxa was found to be effective against the biofilm of any type of normal and clinical strains of
Staphylococcus aureus [
113].
This shows the high potential and effectivity of nanoparticles for treating the infections associated with biofilms [
115]. Attenuation of the signals of bacterial quorum sensing can inhibit infection and can also stop the generation of bacterial virulence. Many research works have been conducted and almost all of them showed that the natural compounds possess more effectiveness over artificially synthesized chemicals regarding their treatment of biofilms and establishing them as anti-quorum-sensing agents.
Especially, flavonoid compounds are highly efficient anti-microbial and antibiofilm compounds. However, due to the very low or no dissolution of the flavonoid molecules and the rare bioavailability, minimal application of flavonoids is found [
116]. Experimental observations revealed that phytochemicals, when mixed with chitosan nanoparticles, significantly decreased the QS activity through the inactivation of AI molecules [
117]. Kaempferol, a flavonoid, is known to possess high-anti-quorum-sensing activity [
118]. The application of the kaempferol and chitosan nanoparticles was analyzed on the basis of their properties of hydrogen bonding, hydrodynamic diameter, antioxidant activity, and amorphous transformation. After this, the inhibition of the quorum-sensing molecules by the nanoparticles in a time-dependent pattern is usually studied [
119]. This measurement is done in a violacein pigment with the help of a biosensor strain
Chromobacterium violaceum CV026, which is again operated by an AI known as acylated homoserine lactone (AHL) [
120]. Kaempferol-loaded sodium tripolyphosphate (TPP)on ChNPshave typical particle sizes and zeta potentials of 190 to 200 nm and +30 to +35 mV, respectively, and can be stored up to 30 days and still successfully inhibit the quorum-sensing molecules, namely, the violacein pigment, in
Chromobacterium violaceum CV026 [
121]. After the success of this method, attempts are being made to use it as a novel antimicrobial chemotherapy. In this process, the kaempferol-encapsulated chitosan nanoparticles play the role of a stable and effective quorum-sensing-dependent antimicrobial, antibacterial, and antibiofilm agent [
122].
Quercetin (QUE), another flavonoid phytocompound that is found in many commonly used medicinal plants [
123] holds strong potential for establishing itself as a QS-inhibiting agent against
Staphylococcus aureus,
Pseudomonas aeruginosa, etc. [
124]. However, the effective laboratory application of quercetin alone has stopped because of its lesser solubility in physiological fluids [
125]. Therefore, many research works convey a solubility increase strategy for quercetin, which is done in the form of an amorphous and stable complex of nanoparticles of quercetin and chitosan [
126]. The preparation of this complex is done using an electrostatic method and it is performed to form a complex involving ionized quercetin components and oppositely charged ChNPs [
127]. In optimal conditions, a quercetin and chitosan nanoparticle complex with a size of roughly 150 to 170 nm shows a payload of about 25 to 30% having 60 to 70% efficiency with a long storage ability. Due to the absence of any adverse side effects, the complex of quercetin and ChNPs can be used for various therapeutic purposes. Such a complex is found to be more effective in the inhibition of quorum sensing than quercetin alone. Although this complex could bring about the increased suppression of quorum-sensing-regulating genes, resulting in the haltingof swimming motility and formation of
Pseudomonas aeruginosa biofilms, it could not suppress the formation of its virulence factor [
46]. The prior inhibition of the production of the biofilm’s swimming motility using the quercetin and ChNPs complex revealed an almost five-fold increase in kinetic solubility [
128].
A new type of ChNPsthat are dually crosslinked with genipin and sodium tripolyphosphate (TPP) display quorum quenching activity [
129].
Trans-cinnamaldehyde (CA) is an intensively studied compound that was shown to inhibit QS activity by decreasing the DNA-binding ability of LuxR while inhibiting acyl-homoserine lactone production. In this work, chitosan-based nanocapsules laden with a high concentration of CA were applied to a transformed
E. coli Top 10 strain fluorescence-based reporter [
14,
130].
5. Mechanism of QS Inhibition Using Functionalized Chitosan Nanoparticles
A nanocapsule is a shell made from a nontoxic polymer that encapsulates an inner liquid core at the nanoscale. These have many uses, including promising medical applications for drug delivery, food enhancement, nutraceuticals, and self-healing materials. The benefits of encapsulation methods are the protection of the drug and/or allied substances from the adverse environment, controlled release, and precision targeting. Hence, chitosan in the form of nanoparticles can exert its antibiofilm activity in a more targeted way. TPP-crosslinked nanoparticles (ionically crosslinked; IC-NPs) show considerable anti-quorum-sensing activity despite their inherent colloidal instability in microbiological media.
It was found that nanocapsules can interact with bacteria via electrostatic interaction, thus effectively delivering the quorum-quenching compound CA to the bacteria. The electrostatic adsorption of the chitosan-coated nanocapsules to the bacterial cell envelope is the mechanism that underpins the observed enhancement of the QS inhibition activity [
131].
The polycationic groups in organic nanoparticles that are used for antimicrobial activity cause cell damage, perhaps via an ion exchange interaction between bacteria and charged polymer surfaces, resulting in the disruption of cellular membranes [
132]. Polycationic nanoparticles can enter into cells via endocytosis, followed by the formation of nanoscale membrane holes, which leads to a final membrane translocation. [
133]. The mechanism of interaction of nanoparticles on the cell surface was also reported in terms of the adsorption and penetration (or disruption) of cell membranes, triggering NP-mediated toxicity. This may include steps such as nanoparticle adhesion at the membrane/water interface, passive membrane translocation, membrane restructuring and leakage, and adhesive lipid extraction [
134]. Nanoparticle translocation into a cell is observed to occur via the outer wrapping, followed by free translocation and inner attachment and embedment [
135]. The adsorption of NPs leads to cell wall depolarization, inducing cellular toxicity and degradation, which allows ions to enter the cytosol. Sometimes, NPs cause irregular pits on the cell wall surface, enabling ions to enter the cell [
136]. The polysaccharides of EPS interact with SO
4 groups of functionalized polystyrene NPs via hydrophobic complexation, which disrupts bacterial biofilm formation [
137].
Quaternary ammonium chitosan NPs can produce long cationic polymer chains that penetrate the cell membrane and can induce ion exchange, which disrupts biofilms [
138].
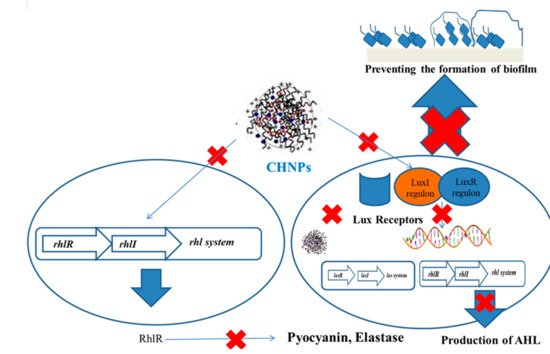
The positive surface of QAS ciprofloxacin-loaded nanochitosan-coated Ti implants disintegrates the negatively charged bacteria, followed by the release of ciprofloxacin, which inhibits enzymes, including DNA gyrase, and topoisomerase causes bacterial disruption. Free radicals interact with endogenous molecular oxygen to produce ROS, superoxide hydroxyl radicals, and hydrogen peroxide, which damages the bacteria membrane integrity and causes irreparable bacteria lysis [
139]. Quaternized-chitosan-loaded Ag NPs release Ag ions that disintegrate the bacteria and inhibit biofilm development [
140] (
Figure 2).
Figure 2. Mechanism of inhibition of biofilm by ChNPs.
This entry is adapted from the peer-reviewed paper 10.3390/polym13152533