The field of nutrition in early life, as an effective tool to prevent and treat chronic diseases, has attracted a large amount of interest over recent years. The vital roles of food products and nutrients on the body’s molecular mechanisms have been demonstrated. The knowledge of the mechanisms and the possibility of controlling them via what we eat has opened up the field of precision nutrition, which aims to set dietary strategies in order to improve health with the greatest effectiveness. However, this objective is achieved only if the genetic profile of individuals and their living conditions are also considered. The relevance of this topic is strengthened considering the importance of nutrition during childhood and the impact on the development of obesity. In fact, the prevalence of global childhood obesity has increased substantially from 1990 and has now reached epidemic proportions.
- pediatrics
- obesity
- cardiometabolic risk factors
- precision nutrition
- eating behavior
1. Introduction
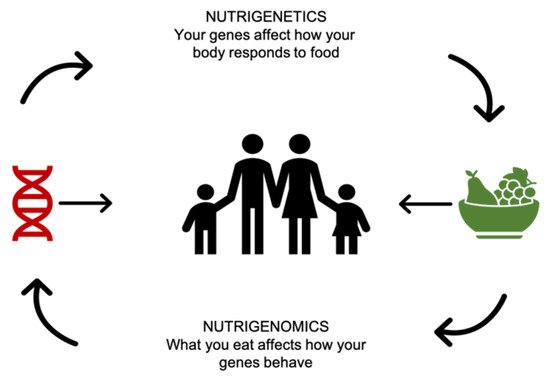
-
“The health effects of nutrients and nutriomes (nutrient combinations) depend on inherited genetic variants that alter the uptake and metabolism of nutrients and/or the molecular interaction of enzymes with their nutrient cofactor and hence the activity of biochemical reactions.”
-
“Nutrition may exert its impact on health outcomes by directly affecting the expression of genes in critical metabolic pathways and/or indirectly by affecting the incidence of genetic mutation at the base sequence or chromosomal level which, in turn, causes alterations in gene dosage and gene expression.”
-
“Better health outcomes can be achieved if nutritional requirements are customized for each individual taking into consideration both his/her inherited and acquired genetic characteristics depending on life stage, dietary preferences, and health status.”
Purpose of Review
2. Precision Nutrition Levels
| Level of Precision Nutrition | Pediatric Stage | |||
|---|---|---|---|---|
| 1. Behavioral Level | 1.1. Pregnancy [8][9][10][11][12][13][14][15][16][17][18] |
1.2. Lactation period [19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50] |
1.3. Childhood and adolescence [51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69] |
|
| First 1000 days | ||||
| 2. Biological Levels | 2.1. Biomarkers [70][71][72][73][74][75][76][77] |
|||
| 2.2. Genetics [78][79][80][81][82][83][84][85][86][87] |
||||
| 2.3. Metabolomics [88][89][90][91] |
||||
| 2.4. Microbiota [92][93][94][95][96][97][98][99][100][101][102][103][104][105][106] |
||||
3. Future Directions
This entry is adapted from the peer-reviewed paper 10.3390/nu12113508
References
- Farpour-Lambert, N.J.; Baker, J.L.; Hassapidou, M.; Holm, J.C.; Nowicka, P.; O’Malley, G.; Weiss, R. Childhood Obesity Is a Chronic Disease Demanding Specific Health Care—A Position Statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO). Obes. Facts 2015, 8, 342–349.
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757.
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236.
- Sharma, P.; Dwivedi, S. Nutrigenomics and Nutrigenetics: New Insight in Disease Prevention and Cure. Indian J. Clin. Biochem. 2017, 32, 371–373.
- Fenech, M.; El-Sohemy, A.; Cahill, L.; Ferguson, L.R.; French, T.A.C.; Tai, E.S.; Milner, J.; Koh, W.P.; Xie, L.; Zucker, M.; et al. Nutrigenetics and nutrigenomics: Viewpoints on the current status and applications in nutrition research and practice. J. Nutr. Nutr. 2011, 4, 69–89.
- Betts, J.A.; Gonzalez, J.T. Personalised nutrition: What makes you so special? Nutr. Bull. 2016, 41, 353–359.
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361.
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417.
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816.
- Ramachenderan, J.; Bradford, J.; McLean, M. Maternal obesity and pregnancy complications: A review. Aust. N. Z. J. Obstet. Gynaecol. 2008, 48, 228–235.
- Tanentsapf, I.; Heitmann, B.L.; Adegboye, A.R. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 2011, 11, 81.
- Mennella, J.A.; Jagnow, C.P.; Beauchamp, G.K. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001, 107, E88.
- Teegarden, S.L.; Scott, A.N.; Bale, T.L. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 2009, 162, 924–932.
- Brion, M.J.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Davey Smith, G.; Lawlor, D.A. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756.
- Murrin, C.; Shrivastava, A.; Kelleher, C.C. Maternal macronutrient intake during pregnancy and 5 years postpartum and associations with child weight status aged five. Eur. J. Clin. Nutr. 2013, 67, 670–679.
- Campbell, D.M.; Hall, M.H.; Barker, D.J.; Cross, J.; Shiell, A.W.; Godfrey, K.M. Diet in pregnancy and the offspring’s blood pressure 40 years later. Br. J. Obstet. Gynaecol. 1996, 103, 273–280.
- Shiell, A.W.; Campbell-Brown, M.; Haselden, S.; Robinson, S.; Godfrey, K.M.; Barker, D.J. High-meat, low-carbohydrate diet in pregnancy: Relation to adult blood pressure in the offspring. Hypertension 2001, 38, 1282–1288.
- Hrolfsdottir, L.; Halldorsson, T.I.; Rytter, D.; Bech, B.H.; Birgisdottir, B.E.; Gunnarsdottir, I.; Granstrom, C.; Henriksen, T.B.; Olsen, S.F.; Maslova, E. Maternal Macronutrient Intake and Offspring Blood Pressure 20 Years Later. J. Am. Heart Assoc. 2017, 6, e005808.
- Wu, Y.; Lye, S.; Dennis, C.L.; Briollais, L. Exclusive breastfeeding can attenuate body-mass-index increase among genetically susceptible children: A longitudinal study from the ALSPAC cohort. PLoS Genet. 2020, 16, e1008790.
- Jackson, K.M.; Nazar, A.M. Breastfeeding, the immune response, and long-term health. J. Am. Osteopath. Assoc. 2006, 106, 203–207.
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587–596.e9.
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37.
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267.
- Flores, T.R.; Mielke, G.I.; Wendt, A.; Nunes, B.P.; Bertoldi, A.D. Prepregnancy weight excess and cessation of exclusive breastfeeding: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 480–488.
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunesova, M.; Hejgaard, T.; Garcia Solano, M.; Fijalkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243.
- Nozhenko, Y.; Asnani-Kishnani, M.; Rodriguez, A.M.; Palou, A. Milk Leptin Surge and Biological Rhythms of Leptin and Other Regulatory Proteins in Breastmilk. PLoS ONE 2015, 10, e0145376.
- Tahir, M.J.; Haapala, J.L.; Foster, L.P.; Duncan, K.M.; Teague, A.M.; Kharbanda, E.O.; McGovern, P.M.; Whitaker, K.M.; Rasmussen, K.M.; Fields, D.A.; et al. Higher Maternal Diet Quality during Pregnancy and Lactation Is Associated with Lower Infant Weight-For-Length, Body Fat Percent, and Fat Mass in Early Postnatal Life. Nutrients 2019, 11, 632.
- Li, R.; Fein, S.B.; Grummer-Strawn, L.M. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics 2010, 125, 1386.
- Disantis, K.I.; Collins, B.N.; Fisher, J.O.; Davey, A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int. J. Behav. Nutr. Phys. Act. 2011, 8, 89.
- Brown, A.; Lee, M.D. Early influences on child satiety-responsiveness: The role of weaning style. Pediatr. Obes. 2015, 10, 57–66.
- Brown, A.; Lee, M. Breastfeeding during the first year promotes satiety responsiveness in children aged 18–24 months. Pediatr. Obes. 2012, 7, 382–390.
- Obermann-Borst, S.A.; Eilers, P.H.; Tobi, E.W.; de Jong, F.H.; Slagboom, P.E.; Heijmans, B.T.; Steegers-Theunissen, R.P. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr. Res. 2013, 74, 344–349.
- Miralles, O.; Sanchez, J.; Palou, A.; Pico, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity 2006, 14, 1371–1377.
- Palou, A.; Pico, C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite 2009, 52, 249–252.
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161.
- Trabulsi, J.C.; Smethers, A.D.; Eosso, J.R.; Papas, M.A.; Stallings, V.A.; Mennella, J.A. Impact of early rapid weight gain on odds for overweight at one year differs between breastfed and formula-fed infants. Pediatr. Obes. 2020, 15, e12688.
- Singhal, A.; Kennedy, K.; Lanigan, J.; Fewtrell, M.; Cole, T.J.; Stephenson, T.; Elias-Jones, A.; Weaver, L.T.; Ibhanesebhor, S.; MacDonald, P.D.; et al. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 1133–1144.
- Escribano, J.; Luque, V.; Ferre, N.; Mendez-Riera, G.; Koletzko, B.; Grote, V.; Demmelmair, H.; Bluck, L.; Wright, A.; Closa-Monasterolo, R. European Childhood Obesity Trial Study Group Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int. J. Obes. (Lond.) 2012, 36, 548–553.
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. European Childhood Obesity Trial Study Group Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051.
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845.
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118.
- Kouwenhoven, S.M.P.; Antl, N.; Finken, M.J.J.; Twisk, J.W.R.; van der Beek, E M.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; Schierbeek, H.; Holdt, L.M.; van Goudoever, J.B.; et al. A modified low-protein infant formula supports adequate growth in healthy, term infants: A randomized, double-blind, equivalence trial. Am. J. Clin. Nutr. 2020, 111, 962–974.
- Wood, A.C.; Blissett, J.M.; Brunstrom, J.M.; Carnell, S.; Faith, M.S.; Fisher, J.O.; Hayman, L.L.; Khalsa, A.S.; Hughes, S.O.; Miller, A.L.; et al. Caregiver Influences on Eating Behaviors in Young Children: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2020, 9, e014520.
- Carruth, B.R.; Ziegler, P.J.; Gordon, A.; Barr, S.I. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J. Am. Diet. Assoc. 2004, 104, 57.
- Momin, S.R.; Hughes, S.O.; Elias, C.; Papaioannou, M.A.; Phan, M.; Vides, D.; Wood, A.C. Observations of Toddlers’ sensory-based exploratory behaviors with a novel food. Appetite 2018, 131, 108–116.
- Luchini, V.; Musaad, S.; Lee, S.Y.; Donovan, S.M. Observed differences in child picky eating behavior between home and childcare locations. Appetite 2017, 116, 123–131.
- Horne, P.J.; Tapper, K.; Lowe, C.F.; Hardman, C.A.; Jackson, M.C.; Woolner, J. Increasing children’s fruit and vegetable consumption: A peer-modelling and rewards-based intervention. Eur. J. Clin. Nutr. 2004, 58, 1649–1660.
- Carper, J.L.; Orlet Fisher, J.; Birch, L.L. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite 2000, 35, 121–129.
- Campbell, K.; Andrianopoulos, N.; Hesketh, K.; Ball, K.; Crawford, D.; Brennan, L.; Corsini, N.; Timperio, A. Parental use of restrictive feeding practices and child BMI z-score. A 3-year prospective cohort study. Appetite 2010, 55, 84–88.
- Jansen, P.W.; Tharner, A.; van der Ende, J.; Wake, M.; Raat, H.; Hofman, A.; Verhulst, F.C.; van Ijzendoorn, M.H.; Jaddoe, V.W.; Tiemeier, H. Feeding practices and child weight: Is the association bidirectional in preschool children? Am. J. Clin. Nutr. 2014, 100, 1329–1336.
- Esposito, K.; Kastorini, C.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12.
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241.
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505.
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 Fatty Acids and Risk for Cardiovascular Disease: A Science Advisory From the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907.
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Stark, R.; Altenhofer, J.; Henze, K.; Parhofer, K. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: A Prospective, Randomized, Controlled Trial. Nutrients 2017, 9, 1097.
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.C.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933.
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265.
- Kirk, S.; Brehm, B.; Saelens, B.E.; Woo, J.G.; Kissel, E.; D’Alessio, D.; Bolling, C.; Daniels, S.R. Role of Carbohydrate Modification in Weight Management among Obese Children: A Randomized Clinical Trial. J. Pediatr. 2012, 161, 320–327.e1.
- Daniels, S.R.; Hassink, S.G. The Role of the Pediatrician in Primary Prevention of Obesity. Pediatrics 2015, 136, 275.
- Gow, M.L.; Ho, M.; Burrows, T.L.; Baur, L.A.; Stewart, L.; Hutchesson, M.J.; Cowell, C.T.; Collins, C.E.; Garnett, S.P. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: A systematic review. Nutr. Rev. 2014, 72, 453–470.
- Garvey, W.T.; Mechanick, J.I. Proposal for a Scientifically-Correct and Medically-Actionable Disease Classification System (ICD) for Obesity. Obesity 2020, 28, 484–492.
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136.
- Hebebrand, J.; Holm, J.; Woodward, E.; Baker, J.L.; Blaak, E.; Schutz, D.D.; Farpour-Lambert, N.J.; Frühbeck, G.; Halford, J.G.C.; Lissner, L.; et al. A Proposal of the European Association for the Study of Obesity to Improve the ICD-11 Diagnostic Criteria for Obesity Based on the Three Dimensions Etiology, Degree of Adiposity and Health Risk. OFA 2017, 10, 284–307.
- Kim, J.; Kim, Y.; Seo, Y.; Park, K.; Jang, H.B.; Lee, H.; Park, S.I.; Lim, H. Evidence-based customized nutritional intervention improves body composition and nutritional factors for highly-adherent children and adolescents with moderate to severe obesity. Nutr. Res. Pr. 2020, 14, 262–275.
- Rancourt, D.; McCullough, M. Overlap in Eating Disorders and Obesity in Adolescence. Curr. Diabetes Rep. 2015, 15, 1–9.
- Cena, H.; Stanford, F.C.; Ochner, L.; Fonte, M.L.; Biino, G.; Giuseppe, R.D.; Taveras, E.; Misra, M. Association of a history of childhood-onset obesity and dieting with eating disorders. Eat. Disord. 2017, 25, 216–229.
- Cebolla, A.; Perpiñá, C.; Lurbe, E.; Alvarez-Pitti, J.; Botella, C. Prevalence of binge eating disorder among a clinical sample of obese children. An. Pediatr. 2012, 77, 98–102.
- De Giuseppe, R.; Di Napoli, I.; Porri, D.; Cena, H. Pediatric Obesity and Eating Disorders Symptoms: The Role of the Multidisciplinary Treatment. A Systematic Review. Front. Pediatr. 2019, 7.
- Balantekin, K.N.; Hayes, J.F.; Sheinbein, D.H.; Kolko, R.P.; Stein, R.I.; Saelens, B.E.; Hurst, K.T.; Welch, R.R.; Perri, M.G.; Schechtman, K.B.; et al. Patterns of Eating Disorder Pathology are Associated with Weight Change in Family-Based Behavioral Obesity Treatment. Obesity 2017, 25, 2115–2122.
- Tracy, R.P. ‘Deep phenotyping’: Characterizing populations in the era of genomics and systems biology. Curr. Opin. Lipidol. 2008, 19, 151–157.
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913.
- Astrup, A.; Hjorth, M.F. Classification of obesity targeted personalized dietary weight loss management based on carbohydrate tolerance. Eur. J. Clin. Nutr. 2018, 72, 1300–1304.
- Hjorth, M.F.; Ritz, C.; Blaak, E.E.; Saris, W.H.; Langin, D.; Poulsen, S.K.; Larsen, T.M.; Sørensen, T.I.; Zohar, Y.; Astrup, A. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: Results from 3 randomized clinical trials. Am. J. Clin. Nutr. 2017, 106, 499–505.
- Hjorth, M.F.; Due, A.; Larsen, T.M.; Astrup, A. Pretreatment Fasting Plasma Glucose Modifies Dietary Weight Loss Maintenance Success: Results from a Stratified RCT. Obesity 2017, 25, 2045–2048.
- Gow, M.L.; Garnett, S.P.; Baur, L.A.; Lister, N.B. The effectiveness of different diet strategies to reduce type 2 diabetes risk in youth. Nutrients 2016, 8, 486.
- Larsen, T.M.; Dalskov, S.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113.
- Papadaki, A.; Linardakis, M.; Larsen, T.M.; van Baak, M.A.; Lindroos, A.K.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesová, M.; Holst, C.; et al. The effect of protein and glycemic index on children’s body composition: The DiOGenes randomized study. Pediatrics 2010, 126, 1143.
- Farooqi, S.; O’Rahilly, S. Genetics of obesity in humans. Endocr. Rev. 2006, 27, 710–718.
- Holzapfel, C.; Drabsch, T. A scientific perspective of personalised gene-based dietary recommendations for weight management. Nutrients 2019, 11, 617.
- Loos, R.J. The genetics of adiposity. Curr. Opin. Genet. Dev. 2018, 50, 86–95.
- Livingstone, K.M.; Celis-Morales, C.; Papandonatos, G.D.; Erar, B.; Florez, J.C.; Jablonski, K.A.; Razquin, C.; Marti, A.; Heianza, Y.; Huang, T.; et al. FTO genotype and weight loss: Systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 2016, 354, i4707.
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679.
- Zlatohlavek, L.; Maratka, V.; Tumova, E.; Ceska, R.; Lanska, V.; Vrablik, M.; Hubacek, J.A. Body adiposity changes after lifestyle interventions in children/adolescents and the NYD-SP18 and TMEM18 variants. Med. Sci. Monit. 2018, 24, 7493–7498.
- Hinney, A.; Wolters, B.; Pütter, C.; Grallert, H.; Illig, T.; Hebebrand, J.; Reinehr, T. No impact of obesity susceptibility loci on weight regain after a lifestyle intervention in overweight children. J. Pediatric Endocrinol. Metab. 2013, 26, 1209–1213.
- Scherag, A.; Kleber, M.; Boes, T.; Kolbe, A.; Ruth, A.; Grallert, H.; Illig, T.; Heid, I.M.; Toschke, A.M.; Grau, K.; et al. SDCCAG8 obesity alleles and reduced weight loss after a lifestyle intervention in overweight children and adolescents. Obesity 2012, 20, 466–470.
- Moleres, A.; Rendo-Urteaga, T.; Zulet, M.A.; Marcos, A.; Campoy, C.; Garagorri, J.M.; Martínez, J.A.; Azcona-Sanjulián, M.C.; Marti, A. Obesity susceptibility loci on body mass index and weight loss in spanish adolescents after a lifestyle intervention. J. Pediatr. 2012, 161.
- Hollensted, M.; Fogh, M.; Schnurr, T.M.; Kloppenborg, J.T.; Have, C.T.; Ruest Haarmark Nielsen, T.; Rask, J.; Lund, M.A.V.; Frithioff-Bøjsøe, C.; Johansen, M.Ø.; et al. Genetic Susceptibility for Childhood BMI has no Impact on Weight Loss Following Lifestyle Intervention in Danish Children. Obesity 2018, 26, 1915–1922.
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Fève, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE 2017, 12, e0173615.
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465.
- Garcia-Perez, I.; Posma, J.M.; Gibson, R.; Chambers, E.S.; Hansen, T.H.; Vestergaard, H.; Hansen, T.; Beckmann, M.; Pedersen, O.; Elliott, P.; et al. Objective assessment of dietary patterns by use of metabolic phenotyping: A randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017, 5, 184–195.
- FiHGV. Obesity and abnormal eating behavior across the lifespan. A crosssectional and longitudinal approach of environmental and neurobiological factors (Eat4healthylife). CiberObn, Madrid, Spain. Available online: https://fihgu.general-valencia.san.gva.es/-/investigadores-de-la-fihguv-valencia-participa-en-el-estudio-eae4healthylife-para-la-identificacion-precoz-de-factores-de-riesgo-asociados-a-la-obesid (accessed on 12 November 2020).
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171.
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Salonen, A.; Lahti, L.; Salojarvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230.
- Biesiekierski, J.R.; Jalanka, J.; Staudacher, H.M. Can Gut Microbiota Composition Predict Response to Dietary Treatments? Nutrients 2019, 11, 1134.
- De Filippis, F.; Pellegrini, N.; Laghi, L.; Gobbetti, M.; Ercolini, D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome 2016, 4, 1–6.
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189.
- Mills, S.; Lane, J.A.; Smith, G.J.; Grimaldi, K.A.; Ross, R.P.; Stanton, C. Precision Nutrition and the Microbiome Part II: Potential Opportunities and Pathways to Commercialisation. Nutrients 2019, 11, 1468.
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722.
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984.
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 1–9.
- Pasolli, E.; Truong, D.T.; Malik, F.; Waldron, L.; Segata, N. Machine Learning Meta-analysis of Large Metagenomic Datasets: Tools and Biological Insights. PLoS Comput. Biol. 2016, 12, e1004977.
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094.
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses. Cell. Metab. 2017, 25, 1243–1253.e5.
