Li7La3Zr2O12 (LLZO) is an inorganic garnet type solid electrolyte which has proven to be one of the most promising electrolytes because of its high ionic conductivity at room temperature, low activation energy, good chemical and electrochemical stability, and wide potential window.
- LLZO
- solid-state electrolyte
- first-principles computing
- synthesis
- doping
- lithium ionic conductivity
1. Introduction
Among many energy storage devices, lithium-ion battery has been the most commonly used technology because of its advantages including high energy density, long duty cycle, low self-discharge, and light weight [1][2][3]. However, there are some concerns about lithium-ion batteries like: safety issues because of the formation of dendrites, reduced power capability because of the formation of solid electrolyte interface (SEI) from continuous charging and discharging, low ionic conductivity, and high cost compared to other existing rechargeable batteries. Extensive research is being carried out by many groups to resolve these issues aiming to improve the performance of lithium-ion batteries.
Electrolyte is one of the most important parts that determine the performance of lithium-ion batteries. According to the state of form, electrolyte materials can be categorized as liquid electrolytes, polymer electrolytes, ionic electrolytes, and solid electrolytes.
2. Structural Analysis of LLZO
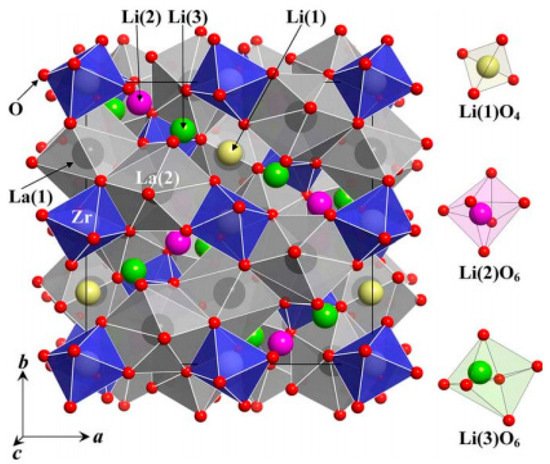
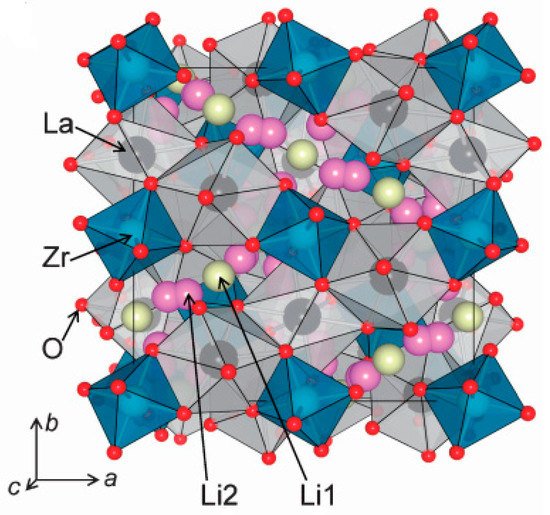
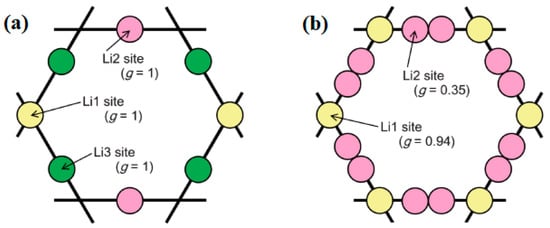
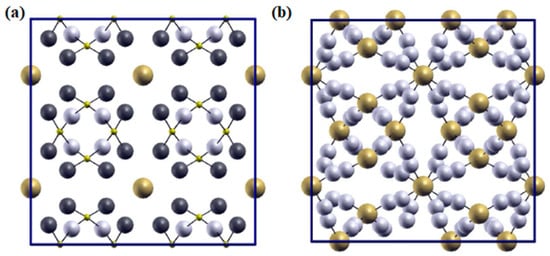
3. Synthesis Techniques of LLZO (Li7La3Zr2O12)
The first LLZO was prepared by Murugan et al. in 2007 using solid state reaction technique where they used sintering temperature of 1230 °C for 36 h [29]. After 2007 this became the most widely used method; however, some researchers reported some concerns regarding this process: (1) the method is difficult in achieving desired stoichiometric structure, (2) high sintering temperature introduces impure phases into the system, (3) the long period of high sintering processing causes too much Li loss from the system, and (4) at high temperature, other foreign elements are introduced from the crucible containing the sample [36][37].
To avoid the problems arising from high sintering temperatures during solid state reaction, which causes Li loss and creates impure phase formation in the system, the sol-gel method has been developed which involves hydrolysis and polymerization. The sol-gel method uses relatively low temperatures and yields uniformly distributed finer sized particles without needing intermittent grinding [38].
The Pechini method is a modified technique of the sol-gel method which involves the ability to chelate metal ions with the help of carboxylic acids such as tartaric acid, citric acid, etc. and forms a polymeric resin due to polysensitization when heated with polyethylene glycol or another hydroxylic alcohol agent. This process requires lower sintering temperature, shorter reaction time and yields uniformly distributed nanosized particles. Using the Pechini method, Gao et al. synthesized Li 5La 3Bi 2O 12 nanocrystalline powder [39], and Jin et al. synthesized stable cubic Al-doped LLZO [40].
Pulsed laser deposition (PLD) has also been used for the fabrication of LLZO. In PLD, a laser beam usually ablates the targets at a suitable angle which helps to preserve the stoichiometry and composition of the target. PLD is very flexible process as it allows the adjustment of conditions that are appropriate for deposition such as the energy of laser density, temperature, and pressure. Also, it reduces the Li loss that occurs during the synthesis process as it allows films to be grown at low temperature in a shorter period. However, a very few studies have been reported so far that used PLD for LLZO preparation. The effect of different substrates on the growth of LLZO at different deposition temperature were investigated by Park et al. [41]. Tan et al. used this method to prepare LLZO on sapphire and SrTiO 3 substrates [42].
4. Sintering Techniques of LLZO
Sintering is one of the most important steps during the fabrication of solid-state electrolytes as it plays a vital role in densifying the pellet of electrolyte. Dense pellets have many advantages such as: (1) decreasing grain boundary resistance and thus decreasing the activation energy to increase the Li ionic conductivity, (2) suppressing the formation of dendrites and accordingly increasing the safe operation of the battery, and (3) increasing the mechanical strength of the battery. Being not dense enough or possessing high porosity in the solid electrolyte increases the chance of fragility, and hence may cause mechanical failure of the device. There are some techniques like furnace sintering [29], hot pressing [37], field assisted sintering technology (FAST) [43] and spark plasma sintering (SPS) [44] reported in the literature that can be used to improve the density of pellets.
Hot pressing involves heat and pressure, where samples are sintered at high temperatures under a certain pressure. Simultaneous application of high temperature and pressure used in this process helps to stabilize the structure and densifies the pellets better. This method is also known as hot isostatic pressing (HIP).
Field-assisted sintering technology (FAST) is a sophisticated modern technology used to sinter samples at a high heating rate. The process of FAST is similar to that of hot isostatic pressing (HIP), however, a dc current passes through the tool along with the uniaxial pressure to assist in sintering process. In other words, the additional heat is generated by an external electrical circuit. FAST has many advantages over other sintering methods in terms of the fast-sintering process that saves time and reduction in Li ion loss that usually occurs due to a long sintering duration. As the synthesis of raw materials and sintering are done together at one time, it avoids the process of tedious intermittent grinding and repeated long-time sintering.
This entry is adapted from the peer-reviewed paper 10.3390/electrochem2030026
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262.
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Mater. Sustain. Energy 2010, 171–179.
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295.
- Gregory, D.H.; O’Meara, P.M.; Gordon, A.G.; Hodges, J.P.; Short, S.; Jorgensen, J.D. Structure of Lithium Nitride and Transition-Metal-Doped Derivatives, Li3-x-yMxN (M = Ni, Cu): A Powder Neutron Diffraction Study. Chem. Mater. 2002, 14, 2063–2070.
- Zintl, E.; Schneider, A. Röntgenanalyse der Lithium-Zink-Legierungen (15. Mitteilung über Metalle und Legierungen). Z. Elektrochem. Angew. Phys. Chem. 1935, 41, 764–767.
- Alpen, U.V.; Rabenau, A.; Talat, G.H. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 1977, 30, 621–623.
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S-GeS2-P2S5 System. J. Electrochem. Soc. 2001, 148, A742–A746.
- Abrahams, I.; Bruce, P.; West, A.; David, W. Structure determination of LISICON solid solutions by powder neutron diffraction. J. Solid State Chem. 1988, 75, 390–396.
- Bruce, P.; West, A. Ionic conductivity of LISICON solid solutions, Li2+2xZn1−xGeO4. J. Solid State Chem. 1982, 44, 354–365.
- Von Alpen, U.; Bell, M.F.; Höfer, H.H. Ionic conductivity in Na4ZrSi3O10. Solid State Ionics 1982, 7, 345–348.
- Hong, H.-P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182.
- Golub, A.; Shumilova, I.; Zubavichus, Y.; Slovokhotov, Y.; Novikov, Y.; Marie, A.; Danot, M. From single-layer dispersions of molybdenum disulfide towards ternary metal sulfides: Incorporating copper and silver into a MoS2 matrix. Solid State Ionics 1999, 122, 137–144.
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. ChemInform Abstract: The Electrical Properties of Ceramic Electrolytes for LiMxTi2-x(PO4)3 + yLi2O, M: Ge, Sn, Hf, and Zr Systems. J. Electrochem. Soc. 1993, 140, 1827.
- Ivanov-Schitz, A. Ionic conductivity of the NaZr2(PO4)3 single crystals. Solid State Ionics 1997, 100, 153–155.
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ionics 2009, 180, 911–916.
- Alamo, J.; Roy, R. Crystal chemistry of the NaZr2(PO4)3, NZP or CTP, structure family. J. Mater. Sci. 1986, 21, 444–450.
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220.
- Sudworth, J. The sodium/sulphur battery. J. Power Sources 1984, 11, 143–154.
- Beevers, C.A.; Ross, Μ.A.S. The Crystal Structure of “Beta Alumina” Na2O·11Al2O3. Z. Krist. Cryst. Mater. 1937, 97, 59–66.
- Farrington, G.; Dunn, B.; Briant, J. Li+ and divalent ion conductivity in beta and beta″ alumina. Solid State Ionics 1981, 3–4, 405–408.
- Bettman, M.; Peters, C.R. Crystal structure of Na2O.MgO.5Al2O3 [sodium oxide-magnesia-alumina] with reference to Na2O.5Al2O3 and other isotypal compounds. J. Phys. Chem. 1969, 73, 1774–1780.
- Bragg, W.L.; Gottfried, C.; West, J. The Structure of β Alumina. Z. Krist. Cryst. Mater. 1931, 77, 255–274.
- Wolfenstine, J.; Allen, J.; Sumner, J.; Sakamoto, J. Electrical and mechanical properties of hot-pressed versus sintered LiTi2(PO4)3. Solid State Ionics 2009, 180, 961–967.
- Wang, B.; Chakoumakos, B.C.; Sales, B.; Kwak, B.; Bates, J. Synthesis, Crystal Structure, and Ionic Conductivity of a Polycrystalline Lithium Phosphorus Oxynitride with the γ-Li3PO4 Structure. J. Solid State Chem. 1995, 115, 313–323.
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ionics 2000, 135, 33–45.
- Catti, M. Local structure of the Li1/8La5/8TiO3(LLTO) ionic conductor by theoretical simulations. J. Phys. Conf. Ser. 2008, 117, 12008.
- Harada, Y.; Ishigaki, T.; Kawai, H.; Kuwano, J. Lithium ion conductivity of polycrystalline perovskite La0.67−xLi3xTiO3 with ordered and disordered arrangements of the A-site ions. Solid State Ionics 1998, 108, 407–413.
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12(M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440.
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781.
- Ramakumar, S.; Janani, N.; Murugan, R. Influence of lithium concentration on the structure and Li+ transport properties of cubic phase lithium garnets. Dalton Trans. 2014, 44, 539–552.
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052.
- Awaka, J.; Takashima, A.; Kataoka, K.; Kijima, N.; Idemoto, Y.; Akimoto, J. Crystal Structure of Fast Lithium-ion-conducting Cubic Li7La3Zr2O12. Chem. Lett. 2011, 40, 60–62.
- Bernstein, N.; Johannes, M.D.; Hoang, K. Origin of the Structural Phase Transition inLi7La3Zr2O12. Phys. Rev. Lett. 2012, 109, 205702.
- Meier, K.; Laino, T.; Curioni, A. Solid-State Electrolytes: Revealing the Mechanisms of Li-Ion Conduction in Tetragonal and Cubic LLZO by First-Principles Calculations. J. Phys. Chem. C 2014, 118, 6668–6679.
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103.
- Liu, K.; Ma, J.-T.; Wang, C.-A. Excess lithium salt functions more than compensating for lithium loss when synthesizing Li6.5La3Ta0.5Zr1.5O12 in alumina crucible. J. Power Sources 2014, 260, 109–114.
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 2012, 206, 28–32.
- Yoo, A.R.; A Yoon, S.; Kim, Y.S.; Sakamoto, J.; Lee, H.C. A Comparative Study on the Synthesis of Al-Doped Li6.2La3Zr2O12 Powder as a Solid Electrolyte Using Sol–Gel Synthesis and Solid-State Processing. J. Nanosci. Nanotechnol. 2016, 16, 11662–11668.
- Gao, Y.; Wang, X.; Wang, W.; Zhuang, Z.; Zhang, D.; Fang, Q. Synthesis, ionic conductivity, and chemical compatibility of garnet-like lithium ionic conductor Li5La3Bi2O12. Solid State Ionics 2010, 181, 1415–1419.
- Jin, Y.; McGinn, P.J. Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J. Power Sources 2011, 196, 8683–8687.
- Park, J.S.; Cheng, L.; Zorba, V.; Mehta, A.; Cabana, J.; Chen, G.; Doeff, M.M.; Richardson, T.J.; Park, J.H.; Son, J.-W.; et al. Effects of crystallinity and impurities on the electrical conductivity of Li–La–Zr–O thin films. Thin Solid Films 2015, 576, 55–60.
- Tan, J.; Tiwari, A. Fabrication and Characterization of Li7La3Zr2O12 Thin Films for Lithium Ion Battery. ECS Solid State Lett. 2012, 1, Q57–Q60.
- Zhang, Y.; Chen, F.; Tu, R.; Shen, Q.; Zhang, L. Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. J. Power Sources 2014, 268, 960–964.
- Kobayashi, Y.; Miyashiro, H.; Takeuchi, T.; Shigemura, H.; Balakrishnan, N.; Tabuchi, M.; Kageyama, H.; Iwahori, T. All-solid-state lithium secondary battery with ceramic/polymer composite electrolyte. Solid State Ionics 2002, 152–153, 137–142.
