Magnetophoresis offers many advantages for manipulating magnetic targets in microsystems. The integration of micro-flux concentrators and micro-magnets allows achieving large field gradients and therefore large reachable magnetic forces. However, the associated fabrication techniques are often complex and costly, and besides, they put specific constraints on the geometries. Magnetic composite polymers provide a promising alternative in terms of simplicity and fabrication costs, and they open new perspectives for the microstructuring, design, and integration of magnetic functions.
- magnetic polymers
- polymer composites
- magnetophoresis
- micro-bead separation
- cell separation
- microfluidic devices
1. Introduction
Microfluidics inspired vivid interest in biomedical applications as it meets a need for the manipulation of micro- and nanoscale objects by offering appealing features. Among them, we can cite: (i) its micrometric dimensions and laminar flow nature, enabling precise object manipulation and single-cell study; (ii) the handling of small quantities of volume, which facilitates the analysis of rare or expensive samples and speeds up processes, leading to cost-effective devices; (iii) the integration of various functions (mixing, focusing, sorting, trapping, detection, etc.) into a single device, leading to compact and portable systems and therefore opening the way for the implementation of point-of-care devices.
The manipulation of micro- and nano-objects requires external forces, such as acoustic, electrical, optical, or thermal actuations. In particular, magnetic forces are suitable for this purpose. Magnetic force-based manipulation relies on magnetophoresis, which refers to the motion of magnetic particles or magnetically labeled cells when subjected to a non-uniform magnetic field. Magnetophoresis [1][2][3][4][5] has been demonstrated as an efficient way to trap and separate biological entities, be it DNA [6][7][8], proteins [9][10][11], beads [12], or cells [13][14][15][16].
Composite polymers have recently emerged as a real breakthrough for the compatible and cost-effective integration of magnetic materials into polymer-based MEMS and microfluidic devices [17][18]. In general, the composite approach allows conferring new properties to the polymers and finds many applications in the field of smart devices [19]. Concerning magnetic composite polymers dedicated to microfluidic systems, this approach enables the tailoring of the magnetic function depending on the nature, the size, the concentration, and the morphology of the magnetic powder, the nature of the polymer matrix, and the microfabrication method. Various polymer materials have been investigated for microfluidic applications: elastomers such as polydimethylsiloxane (PDMS) [20], photosensitive resists such as SU-8 [21], or thermoplastics such as polymethylmethacrylate (PMMA) [22]. Magnetic PDMS is the most commonly encountered due to the microfabrication properties of PDMS by soft lithography and the massive use of the latter for the realization of microfluidic systems. Thus, a large panel of microfluidic functionalities for fluid sample handling has emerged employing composite polymers, such as micro-valves, micro-pumps, or micro-mixers for microfluidic flow control [17][21][23][24][25][26][27][28]; dynamic artificial cilia [22][29][30][31]; and reversible microchannel bonding [32]. Ferrofluids were also explored for actuation in microfluidic systems [33][34][35][36].
Magnetic polymers have also been used in microsystems to manipulate magnetic entities such as labeled cells or magnetic micro-beads by magnetophoresis. Several reviews published in the last few years show the richness of the literature and the vitality of magnetophoretic devices [37][38]. They also reveal that in their large majority, works are based on classical techniques of microelectronic manufacturing.
2. The PDMS Composite Approach
PDMS composites are excellent candidates for the integration of active functions into PDMS microsystems. There are many examples in the literature of dielectrophoretic functions based on conductive PDMS [39][40] and magnetic functions based on magnetic PDMS. Magnetic PDMS composites are mainly obtained by mixing soft (Fe, Ni, and Ni-Fe alloys) or hard (NdFeB, ferrites) magnetic powders with a PDMS mixture (base polymer and curing agent). By modifying the nature, shape, concentration, and organization of the doping particles, it is possible to modulate the magnetic properties of the composite materials. One of the major advantages of these composites is that they preserve some properties of PDMS such as micropatterning by soft lithography and surface activation by O2 for plasma bonding with glass and PDMS substrates. It also allows obtaining magnetic microstructures of several micrometers in thickness and with aspect ratios that are hardly obtained with conventional microfabrication techniques. In addition, the composite microstructure can be directly integrated into the microchannels, in a one-step soft-lithography process, avoiding tedious alignment procedures. This very versatile approach allows localizing the magnetic structures inside the channel or in its close vicinity, underneath or on the sides. Moreover, as the magnetic structures are integrated into PDMS microsystems, the polymer matrix being the same for the whole system, the magnetic function is tightly integrated and does not raise heterogeneous integration issues.
2.1. High Concentrated PDMS Composites
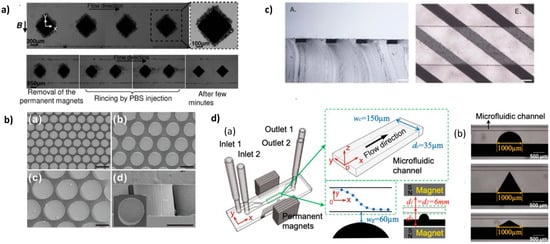
2.2. High Concentrated PDMS Composites with Anisotropic Magnetic Properties
Deman et al. self-organized an 83 wt % (38 vol%) loaded I-PDMS (iron carbonyl/PDMS) composite by applying a 130 mT magnetic field during the polymer cross-linking step [45]. In the non-reticulated polymer, the motion of the magnetic entities is essentially driven by magnetic dipolar interactions. Depending on the relative positions of two adjacent magnetized particles, the interaction can be repulsive or attractive, which leads to anisotropic mechanisms of field-induced structures such as agglomeration and self-organization [46][47][48][49][50].
2.3. Low Concentrated PDMS Composites with Anisotropic Magnetic Properties
2.3.1. Preparation under Uniform Field
A uniform magnetic field induces a uniaxial symmetry in the dipolar interactions. These are attractive along the applied magnetic field direction but repulsive within the normal plane with respect to the applied field direction. Therefore, a uniform field promotes the formation of 1D agglomerates in the volume of the composite. Figure 2 shows the microstructure and the magnetic properties of carbonyl iron particles/PDMS composite (I-PDMS) when prepared under a uniform magnetic field of 130 mT at different concentrations.
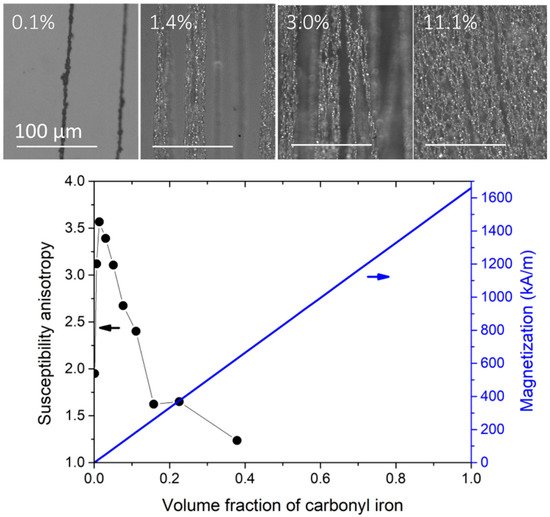
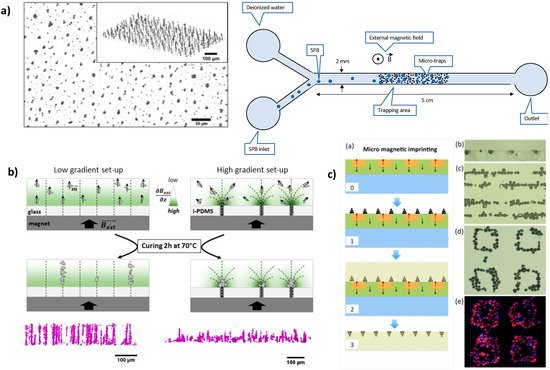
2.3.2. Preparation under Magnetic Field Gradient
3. Magnetic Fluids
An external magnetic field magnetizes the ferrofluid (made of 17 vol% of 10 nm magnetite particles) and thus creates a gradient field (up to 1700 T/m) that attracts magnetic cells (macrophages containing phagocytosed magnetic nanoparticles and intrinsically magnetotactic bacteria) in the side channel (Figure 4a). Thus, the separation efficiency was 10 times increased compared to the separation with a magnet alone.
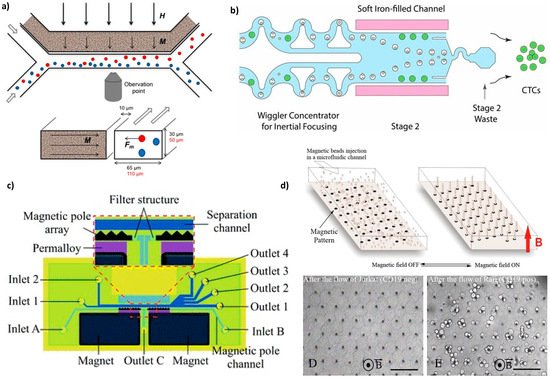
4. Conclusions
PDMS remains the most commonly employed polymer base in magnetic composites. However, thermoplastic polymers, such as polymehtylmethacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC), are commonly used in microfluidics and lab-on-a-chip devices [65][66] and may offer new avenues in the spread of magnetic polymer-based devices. In addition, micro-structured magnetic polymers in microfluidic devices are mainly performed by casting and soft lithography processes, but other printing methods, including 3D printing, are currently explored in soft electronics and soft robotics [67]. These cutting-edge techniques may be investigated by the microfluidic community to implement specific applications, including cell sorting.
This entry is adapted from the peer-reviewed paper 10.3390/magnetochemistry7070100
References
- Munaz, A.; Shiddiky, M.J.A.; Nguyen, N.T. Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 2018, 12, 031501.
- Luo, L.; He, Y. Magnetically driven microfluidics for isolation of circulating tumor cells. Cancer Med. 2020, 9, 4207–4231.
- Deman, A.-L.; Le Roy, D. Magnetophoresis in Bio-Devices. In Engineering of Micro/Nano Biosystems; Springer: Singapore, 2020; pp. 309–361.
- Pamme, N. Magnetism and microfluidics. Lab Chip 2006, 6, 24–38.
- Cao, Q.; Fan, Q.; Chen, Q.; Liu, C.; Han, X.; Li, L. Recent advances in manipulation of micro- and nano-objects with magnetic fields at small scales. Mater. Horiz. 2020, 7, 638–666.
- Hung, P.Y.; Jiang, P.S.; Lee, E.F.; Fan, S.K.; Lu, Y.W. Genomic DNA extraction from whole blood using a digital microfluidic (DMF) platform with magnetic beads. Microsyst. Technol. 2015, 23, 313–320.
- Dias, T.M.; Cardoso, F.A.; Martins, S.A.M.; Martins, V.C.; Cardoso, S.; Gaspar, J.F.; Monteiro, G.; Freitas, P.P. Implementing a strategy for on-chip detection of cell-free DNA fragments using GMR sensors: A translational application in cancer diagnostics using ALU elements. Anal. Methods 2016, 8, 119–128.
- Garbarino, F.; Minero, G.A.S.; Rizzi, G.; Fock, J.; Hansen, M.F. Integration of rolling circle amplification and optomagnetic detection on a polymer chip. Biosens. Bioelectron. 2019, 142, 111485.
- Bejhed, R.S.; Tian, B.; Eriksson, K.; Brucas, R.; Oscarsson, S.; Strömberg, M.; Svedlindh, P.; Gunnarsson, K. Magnetophoretic Transport Line System for Rapid On-Chip Attomole Protein Detection. Langmuir 2015, 31, 10296–10302.
- Zirath, H.; Schnetz, G.; Glatz, A.; Spittler, A.; Redl, H.; Peham, J.R. Bedside Immune Monitoring: An Automated Immunoassay Platform for Quantification of Blood Biomarkers in Patient Serum within 20 Minutes. Anal. Chem. 2017, 89, 4817–4823.
- Gao, Y.; Huo, W.; Zhang, L.; Lian, J.; Tao, W.; Song, C.; Tang, J.; Shi, S.; Gao, Y. Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosens. Bioelectron. 2019, 123, 204–210.
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2010, 110, 1518–1563.
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 1–11.
- Zhao, W.; Cheng, R.; Lim, S.H.; Miller, J.R.; Zhang, W.; Tang, W.; Xie, J.; Mao, L. Biocompatible and label-free separation of cancer cells from cell culture lines from white blood cells in ferrofluids. Lab Chip 2017, 17, 2243–2255.
- Cho, H.; Kim, J.; Jeon, C.W.; Han, K.H. A disposable microfluidic device with a reusable magnetophoretic functional substrate for isolation of circulating tumor cells. Lab Chip 2017, 17, 4113–4123.
- McCloskey, K.E.; Chalmers, J.J.; Zborowski, M. Magnetic Cell Separation: Characterization of Magnetophoretic Mobility. Anal. Chem. 2003, 75, 6868–6874.
- Gray, B.L. A Review of Magnetic Composite Polymers Applied to Microfluidic Devices. J. Electrochem. Soc. 2014, 161, B3173–B3183.
- Yunas, J.; Mulyanti, B.; Hamidah, I.; Said, M.M.; Pawinanto, R.E.; Wan Ali, W.A.F.; Subandi, A.; Hamzah, A.A.; Latif, R.; Majlis, B.Y. Polymer-Based MEMS electromagnetic actuator for biomedical application: A review. Polymers 2020, 12, 1184.
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116.
- Li, J.; Zhang, M.; Wang, L.; Li, W.; Sheng, P.; Wen, W. Design and fabrication of microfluidic mixer from carbonyl iron-PDMS composite membrane. Microfluid. Nanofluidics 2011, 10, 919–925.
- Nakahara, T.; Suzuki, J.; Hosokawa, Y.; Shimokawa, F.; Kotera, H.; Suzuki, T. Fabrication of Magnetically Driven Microvalve Arrays Using a Photosensitive Composite. Magnetochemistry 2018, 4, 7.
- Currie, C.E.; Gray, B.L. Bidirectional Magnetic Polymer Membrane Actuators Integrated into Thermoplastic Microfluidics. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 1056–1059.
- Gholizadeh, A.; Javanmard, M. Magnetically Actuated Microfluidic Transistors: Miniaturized Micro-Valves Using Magnetorheological Fluids Integrated with Elastomeric Membranes. J. Microelectromech. Syst. 2016, 25, 922–928.
- Paknahad, A.A.; Tahmasebipour, M. An electromagnetic micro-actuator with PDMS-Fe3O4 nanocomposite magnetic membrane. Microelectron. Eng. 2019, 216, 111031.
- Said, M.M.; Yunas, J.; Bais, B.; Hamzah, A.A.; Majlis, B.Y. The design, fabrication, and testing of an electromagnetic micropump with a matrix-patterned magnetic polymer composite actuator membrane. Micromachines 2017, 9, 13.
- Nakahara, T.; Ueda, Y.; Miyagawa, H.; Kotera, H.; Suzuki, T. Self-aligned fabrication process for active membrane in magnetically driven micropump using photosensitive composite. J. Micromech. Microeng. 2020, 30, 025006.
- Zhou, R.; Surendran, A.N.; Mejulu, M.; Lin, Y. Rapid microfluidic mixer based on ferrofluid and integrated microscale ndfeb-pdms magnet. Micromachines 2020, 11, 29.
- Tang, S.Y.; Zhang, X.; Sun, S.; Yuan, D.; Zhao, Q.; Yan, S.; Deng, L.; Yun, G.; Zhang, J.; Zhang, S.; et al. Versatile Microfluidic Platforms Enabled by Novel Magnetorheological Elastomer Microactuators. Adv. Funct. Mater. 2018, 28, 1–10.
- Rahbar, M.; Shannon, L.; Gray, B.L. Microfluidic active mixers employing ultra-high aspect-ratio rare-earth magnetic nano-composite polymer artificial cilia. J. Micromech. Microeng. 2014, 24, 025003.
- Zhang, S.; Cui, Z.; Wang, Y.; Den Toonder, J.M.J. Metachronal actuation of microscopic magnetic artificial cilia generates strong microfluidic pumping. Lab Chip 2020, 20, 3569–3581.
- Zhang, S.; Zhang, R.; Wang, Y.; Onck, P.R.; Den Toonder, J.M.J. Controlled Multidirectional Particle Transportation by Magnetic Artificial Cilia. ACS Nano 2020, 14, 10313–10323.
- Tsao, C.W.; Lee, Y.P. Magnetic microparticle-polydimethylsiloxane composite for reversible microchannel bonding. Sci. Technol. Adv. Mater. 2016, 17, 2–11.
- Ashouri, M.; Shafii, M.B.; Moosavi, A. Theoretical and experimental studies of a magnetically actuated valveless micropump. J. Micromech. Microeng. 2017, 27, 015016.
- Hsu, M.C.; Alfadhel, A.; Forouzandeh, F.; Borkholder, D.A. Biocompatible magnetic nanocomposite microcapsules as microfluidic one-way diffusion blocking valves with ultra-low opening pressure. Mater. Des. 2018, 150, 86–93.
- Luo, Z.; Evans, B.A.; Chang, C.H. Magnetically Actuated Dynamic Iridescence Inspired by the Neon Tetra. ACS Nano 2019, 13, 4657–4666.
- Luo, Z.; Zhang, X.A.; Evans, B.A.; Chang, C.H. Active Periodic Magnetic Nanostructures with High Aspect Ratio and Ultrahigh Pillar Density. ACS Appl. Mater. Interfaces 2020, 12, 11135–11143.
- Lim, B.; Vavassori, P.; Sooryakumar, R.; Kim, C. Nano/micro-scale magnetophoretic devices for biomedical applications. J. Phys. D Appl. Phys. 2017, 50, 033002.
- Alnaimat, F.; Karam, S.; Mathew, B.; Mathew, B. Magnetophoresis and Microfluidics: A Great Union. IEEE Nanotechnol. Mag. 2020, 14, 24–41.
- Niu, X.; Peng, S.; Liu, L.; Wen, W.; Sheng, P. Characterizing and patterning of PDMS-based conducting composites. Adv. Mater. 2007, 19, 2682–2686.
- Deman, A.L.; Brun, M.; Quatresous, M.; Chateaux, J.F.; Frenea-Robin, M.; Haddour, N.; Semet, V.; Ferrigno, R. Characterization of C-PDMS electrodes for electrokinetic applications in microfluidic systems. J. Micromech. Microeng. 2011, 21, 095013.
- Bae, Y.M.; Jeong, B.; Kim, J.I.; Kang, D.G.; Shin, K.Y.; Yoo, D.W. Array of 3D permanent micromagnet for immunomagnetic separation. J. Micromech. Microeng. 2019, 29, 085007.
- Faivre, M.; Gelszinnis, R.; Terrier, N.; Ferrigno, R.; Deman, A. Magnetophoretic manipulation in microsystem using carbonyl iron-polydimethylsiloxane microstructures. Biomicrofluidics 2014, 8, 054103.
- Royet, D.; Hériveaux, Y.; Marchalot, J.; Scorretti, R.; Dias, A.; Dempsey, N.M.; Bonfim, M.; Simonet, P.; Frénéa-Robin, M. Using injection molding and reversible bonding for easy fabrication of magnetic cell trapping and sorting devices. J. Magn. Magn. Mater. 2017, 427, 306–313.
- Zhou, R.; Wang, C. Microfluidic separation of magnetic particles with soft magnetic microstructures. Microfluid. Nanofluidics 2016, 20, 1–11.
- Deman, A.L.; Mekkaoui, S.; Dhungana, D.; Chateaux, J.F.; Tamion, A.; Degouttes, J. Dupuis, V.; Le Roy, D. Anisotropic composite polymer for high magnetic force in microfluidic systems. Microfluid. Nanofluidics 2017, 21, 170.
- Liu, J.; Lawrence, E.M.; Wu, A.; Ivey, M.L.; Flores, G.A.; Javier, K.; Bibette, J.; Richard, J. Field-induced structures in ferrofluid emulsions. Phys. Rev. Lett. 1995, 74, 2828–2831.
- Martin, J.E.; Venturini, E.; Odinek, J.; Anderson, R.A. Anisotropic magnetism in field-structured composites. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 61, 2818–2830.
- Bertoni, G.; Torre, B.; Falqui, A.; Fragouli, D.; Athanassiou, A.; Cingolani, R. Nanochains formation of superparamagnetic nanoparticles. J. Phys. Chem. C 2011, 115, 7249–7254.
- Wang, M.; He, L.; Yin, Y. Magnetic field guided colloidal assembly. Mater. Today 2013, 16, 110–116.
- Ghosh, S.; Puri, I.K. Changing the magnetic properties of microstructure by directing the self-assembly of superparamagnetic nanoparticles. Faraday Discuss. 2015, 181, 423–435.
- Dempsey, N.M.; Le Roy, D.; Marelli-Mathevon, H.; Shaw, G.; Dias, A.; Kramer, R.B.G.; Viet Cuong, L.; Kustov, M.; Zanini, L.F.; Villard, C.; et al. Micro-magnetic imprinting of high field gradient magnetic flux sources. Appl. Phys. Lett. 2014, 104, 262401.
- Descamps, L.; Mekkaoui, S.; Audry, M.-C.; Deman, A.-L.; Le Roy, D. Optimized process for the fabrication of PDMS membranes integrating permanent micro-magnet arrays. AIP Adv. 2020, 10, 15215.
- Mitrossilis, D.; Röper, J.C.; Le Roy, D.; Driquez, B.; Michel, A.; Ménager, C.; Shaw, G.; Le Denmat, S.; Ranno, L.; Dumas-Bouchiat, F.; et al. Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun. 2017, 8, 13883.
- Bidan, C.M.; Fratzl, M.; Coullomb, A.; Moreau, P.; Lombard, A.H.; Wang, I.; Balland, M.; Boudou, T.; Dempsey, N.M.; Devillers, T.; et al. Magneto-active substrates for local mechanical stimulation of living cells. Sci. Rep. 2018, 8, 1–13.
- Ourry, L.; Le Roy, D.; Mekkaoui, S.; Douillard, T.; Deman, A.L.; Salles, V. Magnetic filaments for anisotropic composite polymers. Nanotechnology 2020, 31, 9.
- Le Roy, D.; Shaw, G.; Haettel, R.; Hasselbach, K.; Dumas-Bouchiat, F.; Givord, D.; Dempsey, N.M. Fabrication and characterization of polymer membranes with integrated arrays of high performance micro-magnets. Mater. Today Commun. 2016, 6, 50–55.
- Le Roy, D.; Dhungana, D.; Ourry, L.; Faivre, M.; Ferrigno, R.; Tamion, A.; Dupuis, V.; Salles, V.; Deman, A.L. Anisotropic ferromagnetic polymer: A first step for their implementation in microfluidic systems. AIP Adv. 2016, 6, 056604.
- Mekkaoui, S.; Descamps, L.; Audry, M.; Deman, A.; Le Roy, D. Nanonewton Magnetophoretic Microtrap Array for Microsystems. Langmuir 2020, 36, 14546–14553.
- Myklatun, A.; Cappetta, M.; Winklhofer, M.; Ntziachristos, V.; Westmeyer, G.G. Microfluidic sorting of intrinsically magnetic cells under visual control. Sci. Rep. 2017, 7, 1–8.
- Mishra, A.; Dubash, T.D.; Edd, J.F.; Jewett, M.K.; Garre, S.G.; Karabacak, N.M.; Rabe, D.C.; Mutlu, B.R.; Walsh, J.R.; Kapur, R.; et al. Ultrahigh-throughput magnetic sorting of large blood volumes for epitope-agnostic isolation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2020, 117, 16839–16847.
- Zeng, L.; Chen, X.; Du, J.; Yu, Z.; Zhang, R.; Zhang, Y.; Yang, H. Label-free separation of nanoscale particles by an ultrahigh gradient magnetic field in a microfluidic device. Nanoscale 2021, 13, 4029.
- Saliba, A.-E.; Saias, L.; Psychari, E.; Minc, N.; Simon, D.; Bidard, F.-C.; Mathiot, C.; Pierga, J.-Y.; Fraisier, V.; Salamero, J.; et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc. Natl. Acad. Sci. USA 2010, 107, 14524–14529.
- Doyle, P.S.; Bibette, J.; Bancaud, A.; Viovy, J.-L. Self-Assembled Magnetic Matrices for DNA Separation Chips. Science 2002, 295, 2237.
- Minc, N.; Fütterer, C.; Dorfman, K.D.; Bancaud, A.; Gosse, C.; Goubault, C.; Viovy, J.L. Quantitative microfluidic separation of DNA in self-assembled magnetic matrixes. Anal. Chem. 2004, 76, 3770–3776.
- Tsao, C.W. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225.
- Gencturk, E.; Mutlu, S.; Ulgen, K.O. Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics 2017, 11, 051502.
- Kim, Y.; Yuk, H.; Zhao, R.; Chester, S.A.; Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279.
