Neuroblastoma is the most common extracranial solid tumour in childhood, accounting for approximately 15% of all cancer-related deaths in the paediatric population. The overall survival of children with high-risk diseases is around 40–50% despite the aggressive treatment protocols. There is an ongoing research effort to increase NB's cellular and molecular biology knowledge to translate essential findings into novel treatment strategies.
- Neuroblastoma
- Monoclonal Antibodies
- Antibody-Drug Conjugates-Based Therapy
- Third-Generation Tyrosine Kinase Inhibitor
- Drug-Loaded Nanoparticles
- cellular immunotherapies
- tumour vaccines
- radiation therapies
- intra-operative treatments
1. Introduction
Neuroblastoma (NB) is the most common extracranial solid tumour in childhood, accounting for approximately 15% of all cancer-related deaths in the paediatric population [1]. It is characterised by heterogeneous clinical behaviour in neonates and often adverse outcomes in toddlers.
The overall survival of children with high-risk disease is around 40–50% despite the aggressive treatment protocols consisting of intensive chemotherapy, surgery, radiation therapy, and hematopoietic stem cell transplantation [2][3].
2. Cellular Immunotherapy
Immunotherapies based on immune effector cells, able to recognise tumour-associated antigens and exert specific cytotoxicity against tumour cells, are promising approaches investigated in several preclinical studies. Chimeric antigen receptor (CAR)—modified T-cells are genetically engineered T-cells that express a synthetic immunoreceptor consisting of an antigen-binding ectodomain (e.g., single-chain Fv (scFv)). This directs them to a particular tumour antigen and signalling domains that trigger T-cell activation and proliferation when the foreign antigen is bound. Specifically, NB cells ubiquitously express the GD2 ganglioside, an attractive tumour-associated antigen for cellular immunotherapy.
[4] engineered Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) to express a chimeric antigen receptor directed to GD2 to see if they would receive optimal co-stimulation after engagement of their receptors, enhancing survival and anti-tumour activity. They found that human virus-specific CAR-CTLs persist in higher numbers and for longer times after administration than T cells expressing the same receptor but lacking viral specificity (CAR-ATCs), with tumour regression or necrosis in half of the subjects tested. Their results show that the long-term low-level presence of CAR-expressing T cells is associated with clinical benefits, and that they can induce complete tumour responses in patients with active NB. However, follow-on studies using a third-generation format of the same CAR-T failed to show significant clinical efficacy in patients [5].
[6] further evaluated this by investigating the anti-NB activity of GD2-specific CAR T-cells combined with bevacizumab (BEV), a specific mAb against vascular endothelial growth factor (VEGFR), in an orthotopic xenograft model of human NB. When combined with BEV, GD2-CAR T-cells massively infiltrated the tumour mass and secreted interferon-γ (IFN-γ), which, in turn, upregulated NB cell expression of PD-L1. Concurrently, tumour infiltrating GD2-CAR T-cells expressed PD-1. PD-L1 silencing or blocking strategies were then advocated to enhance the efficacy of such a combination of therapies.
In fact, these cells are able to recognise phosphoantigens, which are natural nonpeptide phosphorylated intermediates of isoprenoid metabolism operating in human cells. Interestingly, tumour cells express one of them at a high level, the isopentenyl pyrophosphate (IPP), especially when exposed to amino bisphosphonates. [7] showed in preclinical models of NB that the combined treatment with Vδ2+ T-cells (the most common subset of γδ T-cells) and zoledronic acid (ZOL) was able to inhibit tumour cell proliferation and angiogenesis and to induce cell apoptosis, supporting their use as a therapeutic strategy for NB patients. [8] showed that the combination of adoptively transferred Vδ2+ T-cells, expanded in vitro with ZOL and IL-2, with dinutuximab and systemic ZOL suppressed tumour growth compared to antibody or γδT cell-free controls in an immunodeficient mouse model of small established GD2-expressing NB tumours.
Regarding the employment of cytotoxic T lymphocytes (CTL) and NK cells for anti-tumour immunotherapy, one of their limits is represented by the downregulation of HLA-class I molecules by NB cells. [9] proved that an increase in NB cell immunogenicity was possible upon their exposure to active NK cells, which sensitise NB cells’ recognition by CTLs. [10], who showed that early repeated injections of polyclonal IL-2-activated NK cells significantly increased the survival and reduced the bone marrow infiltration of NB-bearing NOD/SCID mice. Interestingly, low doses of human recombinant IL-2 or IL-15 further enhanced the therapeutic effects.
Driven by the increasing recognition of the pivotal role of tumour vasculature in the survival and growth of solid tumours, there has been a great interest in developing approaches that target and disrupt the existent tumours’ vessels [11][12]. Loi M et al. The APA is enhanced and active in pericytes associated with tumour blood vessels, and it has been correlated with neoplastic progression [13][14]. What they found in this study is that the combined targeting of both the endothelial and the perivascular cells not only increased the disruption of the endothelial wall but also resulted in a statistically significant enhanced anti-tumour effect [15].
Besides tumour blood vessels, liposomal DXR formulations can also be directed explicitly towards tumour cells. In this regard, NB exposure with DXR-loaded Fab’ fragments of anti-GD2 immunoliposomes in nude mice leads to significantly greater inhibition of cell proliferation (in vitro), and long-term survival rates approaching 100% suggested that total inhibition of the metastatic growth of human NB was happening [16].
Finally, the combined use of liposomal DXR tagged with an NGR-containing peptide, and anti-GD2 mAb has been administered sequentially to target both tumour vessels and cancer cells, obtaining a more significant inhibition of NB tumour growth than each formulation given alone. Apart from DXR, sterically stabilised liposomes loaded with HPR have been developed to improve the encapsulated drug’s therapeutic efficacy, reducing neo-angiogenesis and tumour cell proliferation [17]. Similarly, vascular-targeted-BTZ-loaded liposomal formulations have been employed to effectively inhibit NB growth, minimise side effects, and increase the therapeutic index compared to the free drug [18].
3. Tumour Vaccines
The use of tumour cell-based vaccines represents an attractive way of generating anti-NB immunity without increasing the toxicity associated with current radiotherapy and chemotherapy protocols. The vaccines train the immune system to recognise and destroy NB cells after chemotherapy.
In this regard, Bauer et al. [19] designed a multimodal tumour vaccine consisting of irradiated tumour cells infected with the oncolytic IL-12-expressing HSV-1 virus (M002), which produced a stable and specific immunisation in a murine model of intracranial tumour.
[20] showed that the therapeutic vaccination with neuro-2a cells knock-down for the inhibitor of differentiation protein 2 (Id2- kd) significantly suppressed tumour growth in well-established NB tumours. This anti-tumour effect was even more substantial when combined with checkpoint inhibitors. An increased number of IFN-γ producing CD8+ T-cells and the infiltration of cytotoxic CD8+ T cells within the tumour were responsible for the effect of this novel tumour vaccine strategy.
Moreover, Berger et al. [21] reported that the oral gavage of attenuated Salmonella typhimurium (SL7207), carrying recent generated survivin DNA was able to induce a more robust cellular anti-NB immune response than gene gun application or injection of lentivirally transduced bone marrow-derived dendritic cells (DCs) in a syngeneic mouse model of NB.
Similarly, Fest et al. [22] tested a surviving minigene DNA vaccine (pUS-high) administered using SL7207 as a DNA carrier. It proved that this led to complete NB eradication in over 50% of immunised mice.
Because the first-step enzyme of catecholamine biosynthesis, the human tyrosine hydroxylase (hTH), is an important marker for NB, three DNA vaccine plasmids encoding for human hTHcDNA hTH minigene and hTHcDNA have been administered in combination with IL-12 in syngeneic A/J mice to suppress primary tumour growth and spontaneous metastasis [23].
Gil et al. [24] analysed the ability of therapeutic DC vaccines expressing 47-LDA, a CD166 cross-reactive mimotope of the GD2 ganglioside, to selectively expand adoptively transferred tumour-specific T-cells in lymphodepleted NXS2 NB tumour-bearing syngeneic mice. To deliver the antigenic cassette to the activating Fc gamma receptors, the 47-LDA mimotope was presented to DCs either as a linear polypeptide in conjunction with universal Th epitopes or as a fusion protein with the murine Interestingly, the latter formulation was more effective in the induction of the anti-tumour immune response.
[25] recently published the results of a phase II trial for a bivalent vaccine with escalating doses of the immunological adjuvant OPT-821, combined with oral β-glucan. The patient cohort was composed of 102 patients with high-risk NB in remission. This vaccine’s rationale is that if the patient can make antibodies against the two antigens in the vaccine, they could also selectively kill NB cells by attracting the patient’s white blood cells to kill the NB. Their results show that the vaccine plus b-glucan elicited robust antibody responses in patients and that higher anti-GD2-IgG1 title was associated with improved survival.
Toll like receptor 9 (TLR9) agonists, such as synthetic oligonucleotides containing unmethylated CpG motifs (CpG ODNs), can be used as vaccine adjuvants because they can directly induce the activation and maturation of plasmacytoid DCs and can enhance the differentiation of B cells into antibody-secreting plasma cells [26]. [27] evaluated the anti-tumour activity of CpG-containing c-myb antisense oligonucleotides encapsulated with GD2-targeted liposomes in two murine xenograft models of NB. They demonstrated that both the direct inhibition of cell growth, mediated by decreased c-myb protooncogene expression and the indirect CpG-dependent immune stimulation by the NK cell-mediated lysis of tumour cells, resulted in the inhibition of tumour growth, leading to long-term survival in NB-bearing mice. Moreover, as IL-10 is an immune-regulatory cytokine known to suppress macrophages and DC function, the combined administration of CpG ODN-containing liposomes and Abs against IL-10R has proved to prolong immune system activation, leading to better therapeutic results in NB xenografts [28].
4. Radiation Therapy
Radiation therapy is an essential component of NB treatment and is typically administered to both the primary tumour bed after surgical resection and metastatic sites after induction chemotherapy. However, even after radiation therapy, the loco-regional relapse in these patients is still high, with approximately 50% of children relapsing and bearing a 5-year survival of only 8% [29].
As NB patients are very young, undergo intensive multi-agent chemotherapy, and the tumour is often close to radiation-sensitive organs, PBT represents a promising alternative to conventional radiotherapy, especially for reducing the treatment burden associated with it. It is also feasible with very little acute and early late toxicity in the susceptible cohort of very young NB patients.
Different studies on PBT for NB have recently been published, with patients showing similar demographics and treatment strategies before irradiation. In these studies, PBT was performed on the pre-operative tumour bed with 21.6–24 Gray (Gy). [30] reported a 5-year local control rate of 97% after a median follow-up time of 48.7 months, while Bagley et al. [31] published a 5-year local control rate of 87% after a median follow-up of 60.2 months.
[32] performed a retrospective analysis of children with high- or intermediate-risk NB who had PBT of the primary tumour site performed during the first-line therapy. In 39 patients, radiation was given to the pre-operative tumour bed with or without an additional boost in case of residual tumour (five patients received PBT to the MIBG-avid residual at the primary tumour site at the time of PBT). Although the patients received total doses above 30 Gy, in line with the previously mentioned studies, they did not observe relevant toxicity and tumour control rates were high, both for the primary site and the metastases.
NIR-PIT is a newly developed and highly selective cancer treatment that employs a monoclonal antibody conjugated to a photo-absorber dye (IRDye700DX), which is activated by 690 nm light (Figure 1). It represents a promising anti-tumour strategy capable of enhancing immunotherapy’s therapeutic potential by inducing rapid necrotic/immunogenic cancer cell death. The NIR-PIT, in fact, selectively targets cancer cells and induces anti-tumour host immunity with re-priming and proliferation of T-cells that react against cancer-specific antigens. releases tumour-specific antigens into the TME and promotes dendritic cell (DC) maturation, resulting in the presentation of cancer-specific antigens on DCs to naive T cells.
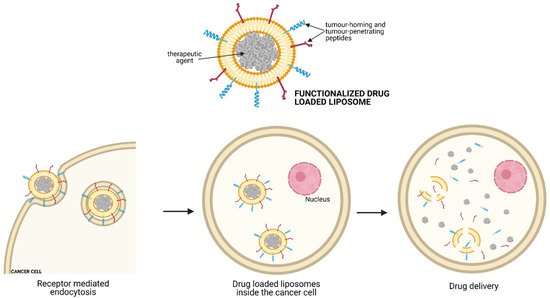
[33] presented the use of NIR-PIT with an anti-GD2-IR700 as a promising anti-tumour strategy to enhance the therapeutic efficacy of anti-GD2 immunotherapy for high-risk NB. They evaluated the anti-tumour effect of anti-GD2-IR700 on three human NB cell lines, showing that the administration of anti-GD2-IR700 significantly suppressed the cell viability compared to anti-GD2 mAb when combined with NIR light irradiation. IL-15 with cancer cell-targeted NIR-PIT could also inhibit tumour growth by increasing anti-tumour host immunity. The use of CD44 as a tumour target is a well-known marker of cancer stem cells as it is expressed on the cell membrane of several cancers.
Most NBs express the noradrenaline transporter molecule and take up metaiodobezylguanidine (mIBG), which can be radiolabelled with either123I or131I. The131I-mIBG therapy is currently used for induction and consolidation treatments, with loco-regional control rates of 84–100% in case of persistent MIBG-avid metastatic sites [34].
was the same as the radiation combined with chemotherapy. However, response rates reported from different relapsed/refractory studies had a great range of variation. to131I-mIBG therapy were of clinical significance, there was no evidence of better long-term outcome as measured by event-free survival (EFS) or overall survival (OS). Although the analysis was heterogeneous from the clinical perspective, there are still open questions and uncertainties: how effective is131I-mIBG?
of131I-mIBG treatment might require combining it with different treatment modalities. The advantage of combining other radiotherapy modalities lies in the ability to achieve higher radiation absorbed tumour doses without compromising the dose-limiting organs of each therapy. [35], who improved the molecular radiotherapy outcome through combination with external beam radiotherapy (EBRT) in a mouse model of NB. This study demonstrates the potential of the combined modality EBRT and131I-mIBG therapy as a useful addition to currently available therapeutic protocols.
This entry is adapted from the peer-reviewed paper 10.3390/children8060482
References
- Maris, J.M. Recent Advances in Neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211.
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 35–47.
- Tas, M.L.; Reedijk, A.M.J.; Karim-Kos, H.E.; Kremer, L.C.M.; Vand de Ven, C.P.; Dierselhuis, M.P.; Van Eijkelenburg, N.K.A.; Van Grotel, M.; Kraal, K.C.J.M.; Peek, A.M.L.; et al. Neuroblastoma between 1990 and 2014 in the Netherlands: Increased incidence and improved survival of high-risk Neuroblastoma. Eur. J. Cancer 2020, 124, 47–55.
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russel, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with Neuroblastoma. Nat. Med. 2008, 14, 1264–1270.
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224.
- Bocca, P.; Di Carlo, E.; Caruana, I.; Emionite, L.; Cilli, M.; De Angelis, B.; Quintarelli, C.; Pezzolo, A.; Raffaghello, L.; Morandi, F.; et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human Neuroblastoma preclinical model. Oncoimmunology 2018, 7, e1378843.
- Di Carlo, E.; Bocca, P.; Emionite, L.; Cilli, M.; Cipollone, G.; Morandi, F.; Raffaghello, L.; Pistoia, V.; Prigione, I. Mechanisms of the Antitumor Activity of Human Vγ9Vδ2 T Cells in Combination with Zoledronic Acid in a Preclinical Model of Neuroblastoma. Mol. Ther. 2013, 21, 1034–1043.
- Fisher, J.P.H.; Flutter, B.; Wesemann, F.; Frosch, J.; Rossig, C.; Gustafsson, K.; Anderson, J. Effective combination treatment of GD2-expressing Neuroblastoma and Ewing’s sarcoma using anti-GD2 ch14.18/CHO antibody with Vγ9Vδ2+ γδT cells. Oncoimmunology 2016, 5, e1025194.
- Spel, L.; Boelens, J.-J.; van der Steen, D.M.; Blokland, N.J.G.; Van Noesel, M.M.; Molenaar, J.J.; Heemskerk, M.H.M.; Boes, M.; Nierkens, S. Natural killer cells facilitate PRAME-specific T-cell reactivity against Neuroblastoma. Oncotarget 2015, 6, 35770–35781.
- Castriconi, R.; Dondero, A.; Cilli, M.; Ognio, E.; Pezzolo, A.; De Giovanni, B.; Gambini, C.; Pistoia, V.; Moretta, L.; Moretta, A.; et al. Human NK cell infusions prolong survival of metastatic human Neuroblastoma-bearing NOD/scid mice. Cancer Immunol. Immunother. 2007, 56, 1733–1742.
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Vascular-targeting therapies for treatment of malignant disease. Cancer 2004, 100, 2491–2499.
- Chaplin, D.J.; Horsman, M.R.; Siemann, D.W. Current development status of small-molecule vascular disrupting agents. Curr. Opin. Investig. Drugs 2006, 7, 522–528.
- Marchiò, S.; Lahdenranta, J.; Schlingemann, R.O.; Valdembri, D.; Wesseling, P.; Arap, M.A.; Hajitou, A.; Ozawa, M.G.; Trepel, M.; Giordano, R.J.; et al. Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell 2004, 5, 151–162.
- Schlingemann, R.O.; Oosterwijk, E.; Wesseling, P.; Rietveld, F.J.; Ruiter, D.J. Aminopeptidase a is a constituent of activated pericytes in angiogenesis. J. Pathol. 1996, 179, 436–442.
- Loi, M.; Marchiò, S.; Becherini, P.; Di Paolo, D.; Soster, M.; Curnis, F.; Brignole, C.; Pagna, G.; Perri, P.; Caffa, I. Combined targeting of perivascular and endothelial tumor cells enhances anti-tumor efficacy of liposomal chemotherapy in Neuroblastoma. J. Control. Release 2010, 145, 66–73.
- Pastorino, F.; Brignole, C.; Marimpietri, D.; Sapra, P.; Moase, E.H.; Allen, T.M.; Ponzoni, M. Doxorubicin-loaded Fab’ Fragments of Anti-disialoganglioside Immunoliposomes Selectively Inhibit the Growth and Dissemination of Human Neuroblastoma in Nude Mice. Cancer Res. 2003, 63, 86.
- Di Paolo, D.; Pastorino, F.; Brignole, C.; Corrias, M.V.; Emionite, L.; Cilli, M.; Tamma, R.; Priddy, L.; Amaro, A.; Ferrari, D.; et al. Combined Replenishment of miR-34a and let-7b by Targeted Nanoparticles Inhibits Tumor Growth in Neuroblastoma Preclinical Models. Small 2020, 16, 1906426.
- Zuccari, G.; Milelli, A.; Pastorino, F.; Loi, M.; Petretto, A.; Parise, A.; Marchetti, C.; Minarini, A.; Cilli, M.; Emionite, L.; et al. Tumor vascular targeted liposomal-bortezomib minimises side effects and increases therapeutic activity in human Neuroblastoma. J. Control. Release 2015, 211, 44–52.
- Bauer, D.F.; Pereboeva, L.; Gillespie, G.Y.; Cloud, G.A.; Elzafarany, O.; Langford, C.; Market, J.M.; Lawrence, S.L. Effect of HSV-IL12 Loaded Tumor Cell-Based Vaccination in a Mouse Model of High-Grade Neuroblastoma. J. Immunol. Res. 2016, 2016, 2568125.
- Chakrabarti, L.; Morgan, C.; Sandler, A.D.; Bai, X. Combination of Id2 Knockdown Whole Tumor Cells and Checkpoint Blockade: A Potent Vaccine Strategy in a Mouse Neuroblastoma Model. PLoS ONE 2015, 10, e0129237.
- Berger, E.; Soldati, R.; Huebener, N.; Hohn, O.; Stermann, A.; Durmus, T.; Lobitz, S.; Zenclussen, A.C.; Christiansen, H.; Lode, H.N.; et al. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for Neuroblastoma. Cancer Lett. 2013, 331, 167–173.
- Fest, S.; Huebener, N.; Bleeke, M.; Durmus, T.; Stermann, A.; Woehler, A.; Baykan, B.; Zenclussen, A.C.; Michalsky, E.; Jaeger, I.S.; et al. Survivin minigene DNA vaccination is effective against Neuroblastoma. Int. J. Cancer 2009, 125, 104–114.
- Huebener, N.; Fest, S.; Hilt, K.; Schramm, A.; Eggert, A.; Durmus, T.; Woehler, A.; Stermann, A.; Bleeke, M.; Baykan, B.; et al. Xenogeneic immunisation with human tyrosine hydroxylase DNA vaccines suppresses growth of established Neuroblastoma. Mol. Cancer Ther. 2009, 8, 2392–2401.
- Gil, M.; Bieniasz, M.; Wierzbicki, A.; Bambach, B.J.; Rokita, H.; Kozbor, D. Targeting a mimotope vaccine to activating Fcgamma receptors empowers dendritic cells to prime specific CD8+ T cell responses in tumor-bearing mice. J. Immunol. 2009, 183, 6808–6818.
- Cheung, I.Y.; Cheung, N.-K.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients with High-Risk Neuroblastoma with Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226.
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204.
- Brignole, C.; Pastorino, F.; Marimpietri, D.; Pagnan, G.; Pistorio, A.; Allen, T.M.; Pistoia, V.; Ponzoni, M. Immune cell-mediated antitumor activities of GD2-targeted liposomal c-myb antisense oligonucleotides containing CpG motifs. J. Natl. Cancer Inst. 2004, 96, 1171–1180.
- Brignole, C.; Marimpietri, D.; Pastorino, F.; Di Paolo, D.; Pagnan, G.; Loi, M.; Piccardi, F.; Cilli, M.; Tradori-Cappai, A.; Arrigoni, G.; et al. Anti-IL-10R antibody improves the therapeutic efficacy of targeted liposomal oligonucleotides. J. Control. Release 2009, 138, 122–127.
- Zhao, Q.; Liu, Y.; Zhang, Y.; Meng, L.; Wei, J.; Wang, B.; Wang, H.; Xin, Y.; Dong, L.; Jiang, X. Role and toxicity of radiation therapy in neuroblastoma patients: A literature review. Crit. Rev. Oncol. Hematol. 2020, 149, 102924.
- Hill-Kayser, C.E.; Tochner, Z.; Li, Y.; Kurtz, G.; Lustig, R.A.; James, P.; Balamuth, N.; Womer, R.; Mattei, P.; Grupp, S.; et al. Outcomes After Proton Therapy for Treatment of Pediatric High-Risk Neuroblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 401–408.
- Bagley, A.F.; Grosshans, D.R.; Philip, N.V.; Foster, J.; McAleer, M.F.; McGovern, S.L.; Lassen-Ramshad, Y.; Mahajan, A.; Paulino, A.C. Efficacy of proton therapy in children with high-risk and locally recurrent neuroblastoma. Pediatr. Blood Cancer 2019, 66.
- Jazmati, D.; Butzer, S.; Hero, B.; Ahmad Khalil, D.; Merta, J.; Baumer, C.; Plum, G.; Fuchs, J.; Koerber, F.; Steinmeier, T.; et al. Proton Beam Therapy for Children with Neuroblastoma: Experiences From the Prospective KiProReg Registry. Front. Oncol. 2021, 10, 617506.
- Nouso, H.; Tazawa, H.; Tanimoto, T.; Tani, M.; Oyama, T.; Sato, H.; Noma, K.; Kagawa, S.; Kobayashi, H.; Noda, T.; et al. Abstract 3831: Development of near-infrared photoimmunotherapy targeting GD2-positive Neuroblastoma. Cancer Res. 2018, 3831.
- Gaze, M.N.; Gains, J.E.; Walker, C.; Bomanji, J.B. Optimisation of molecular radiotherapy with [131I]-meta Iodobenzylguanidine for high-risk Neuroblastoma. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 66–78.
- Corroyer-Dulmont, A.; Falzone, N.; Kersemans, V.; Thompson, J.; Allen, D.P.; Able, S.; Kartsonaki, C.; Malcolm, J.; Kinchesh, P.; Hill, M.A.; et al. Improved outcome of 131 I-mIBG treatment through combination with external beam radiotherapy in the SK-N-SH mouse model of Neuroblastoma. Radiother. Oncol. 2017, 124, 488–495.
