Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Filamentous fungi are known to biosynthesize an extraordinary range of azaphilones pigments with structural diversity and advantages over vegetal-derived colored natural products such agile and simple cultivation in the lab, acceptance of low-cost substrates, speed yield improvement, and ease of downstream processing. Modern genetic engineering allows industrial production, providing pigments with higher thermostability, water-solubility, and promising bioactivities combined with ecological functions.
- natural pigments
- filamentous fungi
- azaphilones
- production
- biotechnological tools
- non-mycotoxigenic strains
- regulatory issues
1. Introduction
Color has been used by mankind since the Neolithic period and has been associated to different people such as purple to the Phoenicians, yellow (annatto) to the Mayans, and to different purposes as henna pigments for body and hair coloring in India. In human history, color gained a powerful status in many daily experiences and key decisions. Some studies show, for example, that preference for blues and reds (at the expense of yellowish and greenish hues) influenced auction prices, as reported for Mark Rothko’s rectangular paintings [1].
Color is also naturally associated with chemosensory perceptions regarding flavor, quality and freshness, highly interfering in product choice [2]. In this way, consumers expect some foods to have specific colors. However, variation and heterogeneousness of natural color in foods initiated the process of adding pigments to maintain color uniformity while granting high coloring power, as well as stability in aqueous phase and in different pH [3].
To date, industry still has not overcome the low availability of natural pigments due to problems such as yield and ethical issues [4] [5]. The synthesis of natural pigments such as beta carotene, riboflavin and cantaxanthin xanthophylls (yellow, orange and red palette) [6] is possible but not competitive enough to feed the market.
Azo dyes can be easily synthesized by diazotation of aromatic amines and became the first-choice colorants in food industry for decades [6], but they have been associated with several diseases, including cancer [7] and behavioral problems in children [8]. These facts led regulatory agencies to ban some synthetic colorants and, consequently, food industry is facing the challenge of developing novel formulations containing natural food coloring agents to provide or complement the color palette of foods.
The replacement of artificially colored products by natural ones is also demanded by a new generation of green-minded consumers seeking for “clean label” and safe ingredients. The boom of groups opting for environmentally friendly consumption and healthy lifestyles led to a big change in food consumer behavior, especially by individuals from the so-called Generation Z (Gen-Z). This group was pointed to account for about 40% of all consumers, the largest consumer market share in 2020 [9].
In this scenario, fungi are highly quoted as alternative sources of naturally derived, healthy, safe, stable and low-cost pigments for food industry applications [11,[10]. One of the most promising classes of fungal pigments in research as industrial pigments are azaphilones, compounds that stand out for their yellow, orange and red colors [11]. This class of fungal secondary metabolites encompasses a large number of compounds of polyketide origin, containing a pyrone-quinone core, a chiral quaternary center and hydroxyl groups as substituents. Orange-colored azaphilones usually possess a heterocycle containing a pyranyl oxygen that is susceptible to aminophilic reactions where the pyran oxygen atom is exchanged for a nitrogen atom derived from peptides, nucleic acids, proteins and others [12]. This exchange alters the absorption of the pigment that goes from orange to red, frequently also altering the biological properties.
Azaphilones research is extremely important and literature reporting new azaphilone derivatives described in the last decades, different fungi sources, and a wide scope of biological activities is comprehensive. However, many issues on industrial scaleup of wet bench fermentative conditions, optimized production, efficient extraction protocols to maximize industrial production and certification of generally recognized as safe (GRAS) strains are areas that still demand research and technological development. An expressive number of works have been addressing the challenge to find a safe, low cost azaphilone source to fit the contemporary demand for edible natural pigments that meet regulatory guidelines. The readiness of fungi-derived red colorants for use in food industry was discussed on an interesting paper by Dufossè [13], while production of yellow pigments by Monascus sp. was addressed by Yang et al. [14].
2. Chemistry, Biological Activities and Biosynthetic Pathways of Recently (2020–2021) Reported Azaphilones
2.1. New Azaphilone Compounds
Two complementary reviews cover a good part of literature about azaphilones from 1932 to 2019. Gao et al. [15] reviewed literature from end of 1932 to September 2012, reporting data on 373 azaphilones of 18 categories and Chen et al. [16] published data on the chemistry and biology of azaphilones, covering 252 compounds predominantly originated from 32 genera of fungi reported between October 2012 to December 2019 [16]. Naturally-derived azaphilones reported by Chen et al. [16] were classified in 13 types: nitrogenated, citrinins, austdiols, deflectins, bulgariolactones, spiro-azaphilones, O-substituted, lactone, hydrogenated, chaetovirins, pulvilloric acid, sclerotiorins, and cohaerins. Azaphilone pigments of atrorosin class produced by Talaromyces atroroseus were reviewed by Isbrandt et al. [17], and Morales-Oyervides et al. [18] reviewed natural colorants produced by fungi from Talaromyces/Penicillium genus [18].
In this section, it is presented a summary of the new compounds reported after December 2019 classified according to the fungal genera source. Despite the great number of 100 new compounds reported from January 2020 to March 2021, the azaphilones were isolated only from nine fungal genera (Aspergillus, Chaetomium, Hypoxylon, Monascus, Muycopron, Penicillium, Phomopsis, Pleosporales, and Talaromyces). The genus Phomopsis was not cited in the latest review and now appeared as fungal endophytic sources of chlorinated azaphilone pigments. At this time, it will be presented the new compounds isolated from each genus (Figures 2–9) displayed according to the species (Table 1).
2.1.1. Azaphilones from Aspergillus Genus
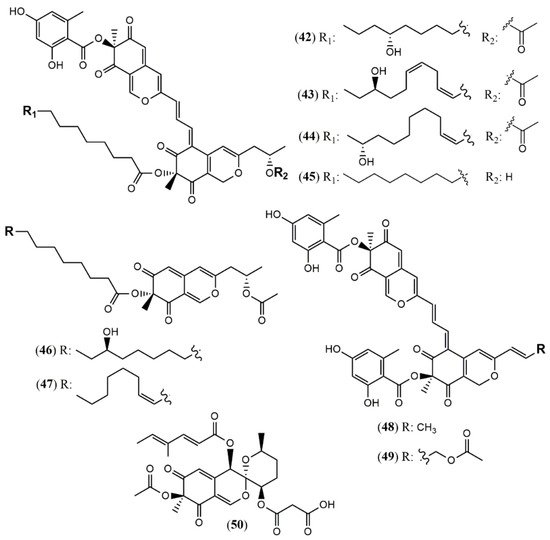
Aspergillus genus is one of the three largest genera where azaphilones can be found. Recently, 23 azaphilones (1–23) were isolated from three species (Figure 2 and Table 1). Sassafrin E (1), sassafrin F (2), and sassafrinamine A (3) were isolated from the filamentous fungus Aspergillus neoglaber 3020 [19]. Two pigments (Sassafrin E (1) and Sassafrin F (2)) were yellow and display the azaphilone core fused to the same angular lactone ring with different substituents. The third pigment (sassafrinamine A) (3) is purple and displays a nitrogen into the isochromene system substituted with ethyl-1-ol group (Figure 2). The fungus Aspergillus cavenicola afforded the nitrogenated azaphilones trans-cavernamine (4), cis-cavernamine (5), amino acid derivatives of cis-cavernamines (6–10), hydroxy-cavernamine (11), amino acid derivatives of hydroxy-cavernamines (12–16), and two oxygenated derivatives cis and trans-cavernines (17–18) [20]. The marine-derived fungus Aspergillus falconensis yielded five mitorubrins derivative azaphilones with different benzoyl moieties: two new chlorinated azaphilones, falconensins O and P (19 and 20) when the fungus was cultivated in a solid rice medium containing 3.5% NaCl and three additional new azaphilone derivatives (21–23) when NaCl was replaced by 3.5% NaBr [21]. From the endophytic Aspergillus terreus of Pinellia ternate, the undescribed dimer of citrinin penicitrinol Q (24) was isolated displaying accentuated Gram-positive antibacterial activity [22].
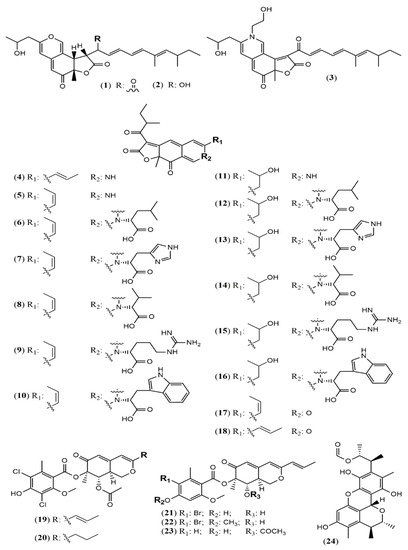
Figure 2. Chemical structures of Aspergillus azaphilones 1–2: sassafrin E-F; 3: sassafrinamine A; 4: trans-cavernamine; 5: cis-cavernamine; 6–10: Leu, His, Val, Arg, Trp-cavernamine derivatives; 11: hydroxy-cavernamines; 12–16: Leu, His, Val, Arg and Trp-hydroxy-cavernamines.; 17: cis-cavernine; 18: trans-cavernine; 19–20: falconensins O and P; 21–23: falconensins Q, R, and S; 24: penicitrinol Q [19][20][21][22].
2.1.2. Azaphilones from Chaetomium Genus
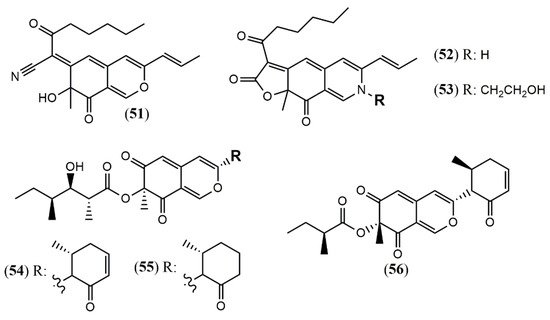
Chaetomium is a large genus presenting more than 300 species worldwide. Chaetomium globosum represents one of the most studied species and is known as a rich source of azaphilones. Since the last two years, this species has still been contributing with new metabolites. The arthropod-associated endophytic fungus C. globosum TW1–1 was investigated considering whether the presence of 1-methyl-l-tryptophan into the growth medium would activate a biosynthetic pathway to produce novel alkaloids [23]. However, instead of nitrogenated metabolites, the authors isolated and identified two chlorinated azaphilones, chaephilone C and D (25–26) with anti-inflammatory activity. Their stereostructures were unequivocally confirmed by X-ray analyses. Nevertheless, chaephilone C was also previously reported from the deep sea-derived fungus Chaetomium sp. NA-S01-R1 with the same planar structure of 25 but with different stereochemistry, suggesting that its structure should be revised [24]. Two months after the report of chaephilone C (25), a new chlorinated azaphilone from C. globosum, endophytic of Polygonatum sibiricum, was reported and also called chaephilone C (27) [25]. However, this latter compound displayed a chemical structure similar to (26), but completely different from the former (25).
From the wild-type strain C. globosum, a new dimeric azaphilone called cochliodone J (28) was identified in the same medium which cochliodone A had been isolated before [26]. The deep-sea C. globosum MP4-S01–7 provided eight new structurally correlated nitrogenated azaphilones 29–36 (Figure 3 and Table 1) [27]. The azaphilone core is the same in all compounds with differences only in the lactone acyl substituents and the N-alquil groups. Seco-chaetomugilin (37) was isolated for the first time from the ethyl acetate extract of Chaetomium cupreum in a bio-guided fractionation for activities against human breast adenocarcinoma cell lines [28]. Although the authors named the compound isolated as seco-chaetomugilin, it presented the same structure of seco-chaetomugilin D, previously isolated from C. globosum [29]. A screening by LC-MS/MS-GNPS data base of a strain of an endophytic plant fungus Chaetomium sp. g1 resulted in the isolation of chaetolactam A (38), a unique 9-oxa-7-azabicyclo[4.2.1]octan-8-onering system with two new compounds chaetoviridins derivatives, 11-epi-chaetomugilide B (39), and chaetomugilide D (40) [30]. Another plant endophytic fungus C. globosum isolated from the desert Asteraceae species, Artemisia desterorum, yielded globosumone (41), a new stereoisomer of the known chaetoviridin E [31].
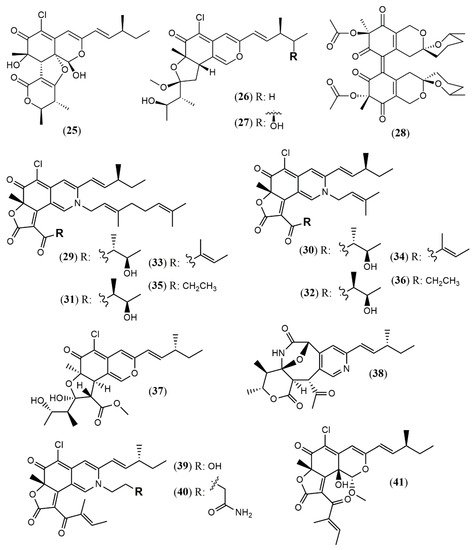
Figure 3. Chemical structures of Chaetomium azaphilones: 25: Chaephilone C (1R,7S,8R,8aR,9E,11S,4′R,5′R); 26: chaephilone D; 27: chaephilone C*; 28: cochliodone J; 29: N-(3,7-Dimethyl-2,6-octadienyl)-2-aza-2-deoxychaetoviridin A; 30: 4′-epi-N-(3,7-Dimethyl-2,6-octadienyl)-2-aza-2-deoxychaetoviridin A; 31: N-(3-Methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A, 32: 4′-epi-N-(3-Methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A; 33: N-(3,7-Dimethyl-2,6-octadienyl)-2-aza-2-deoxychaetoviridin E; 34: N-(3-Methyl-2-butenyl)-2-aza-2-deoxychaetoviridin E; 35: 4′,5′-dinor-5′-Deoxy-N-(3,7-dimethyl-2,6-octadienyl)-2-aza-2- deoxychaetoviridin A; 36: 4′,5′-dinor-5′-Deoxy-N-(3-methyl-2-butenyl)-2-aza-2-deoxy- chaetoviridin A; 37: seco-chaetomugilin; 38: chaetolactam A; 39: 11-epi-chaetomugilide B; 40: chaetomugilide D; 41: globusumone [23][24][25][26][27][28][29][30][31].
2.1.3. Azaphilones from Hypoxylon Genus
Four unprecedented bisazaphilones hybridorubrins A–D (42–45) were isolated together with two new mitorubrin-type azaphilones, fragirubrins F–G (46–47) [32] from Hypoxylon fragiforme. The main differences among them are the acyl substituents in the lenormandin/fragirubrin-type moiety. In this study, the authors determined the azaphilones stereochemistry by electronic circular dichroism (ECD) spectroscopy in a comparative study between isolated and synthetic compounds. The acquired data suggest that the previous stereochemistry reported for rutilins C (48), D (49) and the mitorubrins [33] must be revised to be (S)-configured at C-8 and C-8a (Figure 4). Another species Hypoxylon fuscum complex yielded a new daldinin F derivative possessing a 3′-malonyl group (50) [34].
2.1.4. Azaphilones from Monascus Genus
Monascus pilosus BCRC 38072, a citrinin-free strain, was able to produce several azaphilone pigments including three new Monascus red pigments without citrinin presence: monapilonitrile A (51), monapilosine (52), and N-ethanolic monapilosine (53) [35] (Figure 5). Metabolites (52) and (53) are nitrogenated azaphilones lacking or bearing the N-hydroxyethyl group, respectively.
2.1.5. Azaphilones from Muyocopron Genus
The chemical investigation of the endophyte Muyocopron laterale ECN279 isolated from a health leaf of Conavalia lineata led to the isolation of the two new azaphilones muyocopronones A and B (54–55) [36]. An endophyte fungus F53 from the traditional Chinese medicine plant Taxus yunnanensis had its genome sequenced and mined, and the multi-locus phylogeny of F53 allowed its placement within the genus Muyocopron with its closest relative being Muyocopron atromaculans (MUCL 34983) [37]. Moreover, a new azaphilone lijiquinone 1 (56) with activities against human myeloma cells and the yeast Candida albicans and Cryptococcus albidus was isolated from its ethyl acetate extract (Figure 5).
2.1.6. Azaphilones from Penicillium Genus
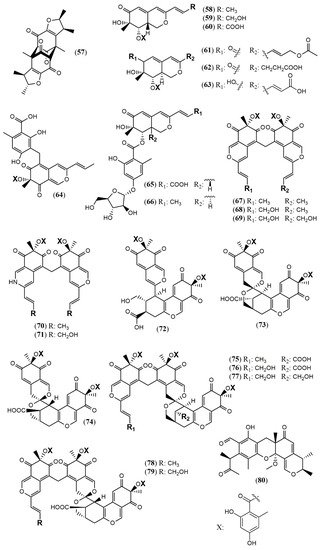
The Penicillium genus produces a great number of azaphilone metabolites [16]. Penicillium citrinum WK-P9 was isolated as an associated fungus from the sponge Suberea sp., displaying antibacterial activity. The bio-guided chemical investigation of its ethyl acetate extract led to the isolation of a new citrinin derivative called penicitrinone G (57) [38]. Genome mining, epigenetic regulation, optimization of culture conditions, and one-strain-many-compounds (OSMAC) were investigated as a possible way to prioritize the production of other polyketide metabolites different than the rubratoxins in Penicillium dangeardii [39]. Only the metabolic shunting strategy, based on the deletion of the key gene rbtJ encoding PKS for rubratoxins biosynthesis, and the optimization of culture conditions successfully led to the production of 35 azaphilones, from which 23 were new ones. They were identified as nine monomers named dangelones A–G (58–64), dangeloside A–B (65–66), eight dimers, didangelones A–G (67–74), and five trimers, tridangelones A–E (75–79) [39] (Figure 6). Dangelones A–G (58–64) have the same planar structure and the distinctions among them lay on the side chains at C-3. The differences at C-3 side chain are also present in the dimers. Still regarding Penicillium endophytic fungi, a strain of Penicillium sp. T2–11 isolated from the rhizomes of the underground portion of Gastrodia elata produced a citrinin dimer, named penctrimertone (80) [40].
2.1.7. Azaphilones from Phomopsis Genus
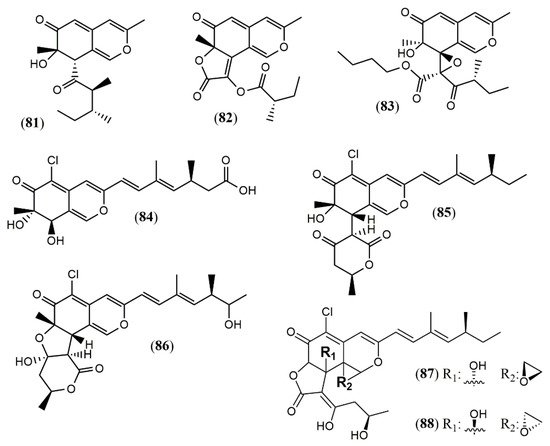
Culture of the endophyte fungus Phomopsis sp. CGMCC No.5416 yielded the three azaphilones phomopsones A–C (81–83), presenting anti-HIV and cytotoxic activity [41]. From the deep-sea-derived fungus Phomopsis tersa FS441, five chlorinated azaphilones named tersaphilones A–E (84–88) presenting unique structures were isolated [42] (Figure 7).
2.1.8. Azaphilones from Pleosporales Genus
The marine-derived fungus Pleosporales sp. CF09–1 produced the uncommon bisazaphilones dipleosporalones A and B (89–90) (Figure 8) [43]. These compounds own a 6/4/6 ring system that might come from a [2 + 2] cycloaddition reaction between two pinophilin B-type monomers and represents the first example of this coupling.
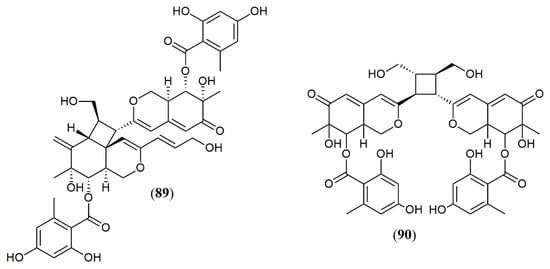
Figure 8. Chemical structures of azaphilones from Pleosporales: 89–90: pleosporales A and B [43].
2.1.9. Azaphilones from Talaromyces Genus
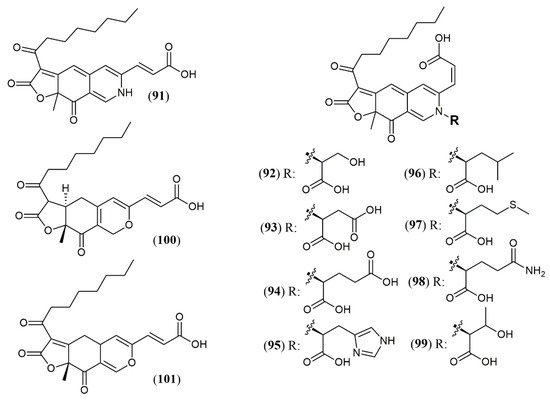
Most strains previously referred to as Penicillium sp. are now classified in the Talaromyces species, and some of them have been found to produce yellow and red azaphilone pigments. Two new pigments from T. atroroseus were described. The first belongs to the series of known Monascus orange azaphilone PP-O pigments, and it was unequivocally elucidated as the isomer trans-PP-O (91) [17] (Figure 9). The second was the unique azaphilone atrosin S, which presented the incorporation of a serine moiety into the isochromene/isoquinoline system. The fungus cultivation in medium enriched with a specific amino acid as sole source of nitrogen could allow seven atrorosin derivatives (atrorosin D, E, H, L, M, Q, and T, depending on the amino acid incorporated) (92–99), which were identified by dereplication using HPLC-DAD-MS/HRMS analysis [17]. From the fungus Talaromyces albobiverticillius associated with the isopod Armadillidium vulgare, two interesting azaphilone pigments talaralbols A and B (100–101) was reported [44]. However, talaralbol B presents the same planar structure of trans-PP-O, early described in T. atroroseus [17], in which the C-9 stereochemistry was not reported.
2.2. Biological Activities of Azaphilones
Azaphilones, besides being good compounds to replace synthetic pigments, aggregate valuable pharmacological properties. The wide broad range of biological activities that has been reported for azaphilones such as cytotoxic, anti-inflammatory, antimicrobial, antitumor, antiviral and antioxidant is exemplified in Table 1.
Concerning the activities regarded to the new 101 azaphilones reported, the cytotoxic and antitumor potential are the most evaluated. Remarkably, compounds (29), (30), and (33) showed the most effective anti-gastric cancer activities (MGC803 and AGS cell lines) with IC50 values less than 1 μM, being more active than the positive control paclitaxel (3.8 μM) [27]. Additionally, (29) and (30) induced apoptosis in a concentration-dependent manner and (30) inhibited cell cycle progression. The authors also claim that 3,7-dimethyl-2,6-octadienyl group attached to N-2 contributed to the potent cytotoxic activities against MGC803 and AGS gastric cancer cell lines what can induce new investigations with semi-synthetic azaphilone derivatives possessing this group [18]. The azaphilones (39) and (40) showed moderate activity against leukemia HL-60 and human breast cancer. However, (39) exhibited potent apoptosis induction activity by mediating caspase-3 activation and PARP degradation at 3 μM in leukemic cells HL-60 [30]. Another interesting result was the potent cytotoxic activity showed by the dimeric azaphilones (89) and (90) against five different human cell lines. (89) showed more potent cytotoxicity against MGC-803 than cisplatin and possessed a unique 6/4/6 ring system suggesting the new ring may play an important role in cytotoxicity [43].
A great number of azaphilones present anti-inflammatory activity [21][23][24][28][35][36][38][39][44][45]. The compounds (21), (51), (52) and (100) exhibited anti-inflammatory activities due to potent anti-NO production activity, with IC50 values of 11.9, 2.6, 12.5, and 10.0 μM, respectively, compared to the known iNOS inhibitor quercetin (34.6 ± 1.4 μM) on lipopolysaccharide (LPS) -induced nitric oxide (NO) production [21][32][43]. The antimicrobial activity of azaphilones also must be highlighted. Two dimeric azaphilones, penicitrinol Q (24) and penctrimertone (80), showed both excellent inhibitory activities against B. subtilis with MIC of 6.2 and 4.0 µg/mL, respectively. Moreover, (24) also presented inhibitory activity against bacteria Staphylococcus aureus (4.3 µg/mL) and Pseudomonas aeruginosa (11.2 µg/mL), and the yeast C. albicans (4.0 µg/mL) [22].
In vitro antiviral activity against HIV-1 was detected for phomopsones B and C (82–83) (7.6 and 0.5 μM, respectively [38]). Research in antiviral potential of azaphilones may be strengthened as they have been focused as possible drug leads for the development of effective antiviral agents against SARS-CoV-2 [46][47]. This worldwide impact-generated virus draws attention to the difficulty in developing new non-toxic antiviral drugs, as viruses use cell host metabolism for replication. This is corroborated by previous reports of antiviral activity of azaphilone metabolites, such as chermisinone B, isolated from the endophytic fungus Nigrospora sp. YE3033, and active against A/Puerto Rico/8/34 (H1N1) in CPE assay (IC50 0.80 μg/mL) with low cellular toxicity on MDCK cells (CC50 184.75 μg/mL) [48]. In vitro HIV-1 replication inhibitory effects in C8166 cells were demonstrated for Helotialins A and B (EC50 8.01 and 27.9 nM, respectively) [49]. In 2019, comazaphilone D was reported as a non-competitive inhibitor of neuraminidase from recombinant rvH1N1 (IC50 30.9 µM) while rubiginosin A was active against H5N1 (IC50 29.9 µM) [50]. The previous knowledge of the antiviral potential of azaphilone derivatives is an advantageous background for the development of new drugs to inhibit SARS-CoV-2.
Table 1. Azaphilones fungal sources and reported biological activities.
| Name (No). | Producing Strains | Activity |
|---|---|---|
| Aspergillus | ||
| Sassafrin E-F (1–2) | A. neogabler IBT3020 [19] |
Data not reported |
| Sassafrinamine A (3) | ||
| Trans-cavernamine(4) | A. cavernicola [20] | Data not reported |
| Cis-cavernamine (5) | ||
| Cis-cavernamines-Leu, His, Val, Arg, Trp (6–10) |
||
| Hydroxy-cavernamine (11) | ||
| Hydroxy-cavernamines-Leu, His, Val, Arg, Trp (12–16) |
||
| Cis-cavernines (17) | ||
| Trans-cavernines (18) | ||
| Falconensins O (19) | A. falconensis [21] | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 15.7 µM) |
| Falconensins P (20) | Not tested | |
| Falconensins Q (21) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 11.9 µM) | |
| Falconensins R (22) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 14.6 µM) | |
| Falconensins S = 8-O-Acetil-falconensin I (23) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 20.1 µM) | |
| Penicitrinol Q (24) | A. terreus [22] | Antimicrobial (S. aureus: 4.3 mg/mL; B. subtilis: 6.2 mg/mL) |
| Chaetomium | ||
| Chaephilone C (1R,7S,8R,8aR,9E, 11S,40R,50R) (25) |
C. globosum TW1–1 [23] |
Anti-inflammatory (inhibit NO production: 15.12 µM) |
| Chaephilone D (26) | Anti-inflammatory (inhibit NO production: 20.65 µM) | |
| Chaephilone C * (27) | C. globosum [25] | Cytotoxic (HepG-2: 38.6 µM); BST (68.6% of letality at 10 mg/mL) |
| Cochliodone J (28) | C. globosum [26] | Cytotoxic (HeLa: 17.3 µM) |
| (4′R,5′R,7S,11S)-N-(3,7- dimethyl-2,6- octadienyl)-2-aza- 2-deoxychaetoviridin A (29) |
C. globosum MP4-S01–7 [27] |
Antitumor (MGC803 and AGS gastric cells lines: 0.78 and 0.12 µM, induced apoptosis) |
| 4′-epi-N-(3,7-dimethyl-2,6-octadienyl)-2-aza-2- deoxychaetoviridin A (30) | Antitumor (MGC803 and AGS gastric cells lines: 0.46 and 0.62 µM, induced apoptosis an altered the cell cycle distribution) | |
| N-(3- methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A (31) | Antitumor (MGC803 and AGS gastric cells lines: 2.7 and 6.5 µM) | |
| 4′- epi-N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A.(32) | Antitumor (MGC803 and AGS gastric cells lines: 3.0 and 2.9 µM) | |
| N-(3,7-dimethyl-2,6- octadienyl)-2-aza-2-deoxychaetoviridin E (33) | Antitumor (MGC803 and AGS gastric cells lines: 0.72 and 0.12 µM) | |
| N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin E (34) | Antitumor (MGC803 and AGS gastric cells lines: 6.8 and 2.0 µM) | |
| 4′,5′-dinor-5′-deoxy-N-(3,7- dimethyl-2,6-octadienyl)-2-aza-2-deoxychaetoviridin A (35) | Antitumor (MGC803 and AGS gastric cells lines: 2.2 and 1.2 µM) | |
| 4′,5′-dinor-5′- deoxy-N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A (36) | Antitumor (MGC803 and AGS gastric cells lines: 5. 8 and >10 µM) | |
| Seco-chaetomugilin (37) | C. cupreum [28] | Anticancer (MCF-7: 75.25% at 50 mg/mL) Increased ROS production: 19.6% at 5 mg/mL |
| Chaetolactam A (38) | Chaetomium sp. g1 [30] |
Cytotoxic (Not detected) |
| 11-epi-chaetomugilide B (39) | Cytotoxic (HL-60: .3.19 µM; A549: 8.37 µM; MCF-7: 4.65 µM; SW480: 4.21 µM; apoptosis induction mediated by caspase 3 in HL-60 cell: 3 µM) | |
| Chaetomugilide D (40) | Cytotoxic (HL-60: .15.92 µM; MCF-7: 17.97 µM; SW480: 14.09 µM; apoptosis induction mediated by caspase 3 in HL-60 cell: 15 µM) | |
| Globosumone (41) | C. globosum [31] | Cytotoxic (Not detected) |
| Hypoxylon | ||
| Hybridorubrin A (42) | H. fragiforme [33] | Antimicrobial (% biofilm inhibition of S. aureus: 81% at 250 mg/mL) |
| Hybridorubrin B (43) | No antimicrobial or cytotoxic activity | |
| Hybridorubrin C (44) | Antimicrobial (% biofilm inhibition of S. aureus: 82% at 250 mg/mL) | |
| Hybridorubrin D (45) | Antimicrobial (% biofilm inhibition of S. aureus: 71% at 250 mg/mL) | |
| Fragirubrin F (46) | Not tested | |
| Fragirubrin G (47) | Not tested | |
| Rutilin C (48) | Antimicrobial (% biofilm inhibition of S. aureus: 58% at 250 mg/mL) | |
| Rutilin D (49) | Not tested | |
| 3′-Malonyl-daldinin F (50) | H. fuscum [34] | Cytotoxic (L929 murine fibroblast: weak; KB 3.1 cervix-cancer cells: weak) |
| Monascus | ||
| Monapilonitrile (51) | M. pilosus BCRC 38072 [35] | Anti-inflammatory (inhibit NO production: 2.6 µM) |
| Monapilosine (52) | Anti-inflammatory (inhibit NO production: 12.5 µM) | |
| N-Ethanolic monapilosine (53) | Anti-inflammatory (inhibit NO production: 27.5 µM); cytotoxic (LPS-induced RAW264.7: cell viability< 65% at 50 µM) | |
| Muyocopron | ||
| Muyocopronone A (54) | M. laterale ECN279 [36] | Antimicrobial (Not detected) |
| Muyocopronone B (55) | Antimicrobial (methicillin-resistant S. aureus and vancomycin-resistant E. faecalis: MIC at 128 mg/mL) | |
| Lijiquinone 1 (56) | Muyocopron sp. ** [37] |
Antifungal (C. albicans: 79 µM; C. albidus: 141 µM); Cytotoxic (RPMI-8226: 129 µM) |
| Penicillium | ||
| Penicitrinone G (57) | P. citrinum WK-P9 [38] |
Antimicrobial (Not detected) |
| Dangelone A (58) | P. dangeardii [39] | Cytotoxic (Inactive: IC > 20 mmol) |
| Dangelone B (59) | Cytotoxic (HepG2: 6.82 mmol; MCF-7: 14.98 mmol) | |
| Dangelone C-G (60–64) | Cytotoxic (Inactive: IC > 20 µM) | |
| Dangeloside A and B (65 and 66) | Cytotoxic (Inactive: IC > 20 µM) | |
| Didangelone A-H (67–74) | Cytotoxic (Inactive: IC > 20 µM) | |
| Tridangelone A-E (75–79) | Cytotoxic (Inactive: IC > 20 µM) | |
| Penctrimertone (80) | Penicillium sp. T2–11 [40] |
Antimicrobial (C. albicans: 4mg/mL; B. subtilis: 4mg/mL); cytotoxic (HL-60: 16.77 µM; SMMC-7721: 23.03 µM; A-549: 28.62 µM; MCF-7: 21.53 µM) |
| Phomopsis | ||
| Phomopsone A (81) | Phomopsis sp. CGMCC No.5416 [41] |
Antiviral (Not detected); cytotoxic (Not detected) |
| Phomopsone B (82) | Antiviral (HIV-1: 7.6 µM); cytotoxic (A549: 176.7 µM; MDA-MB-231: 303.0 µM); | |
| Phomopsone C (83) | Antiviral (HIV-1: 0.5 µM); cytotoxic (A549: 8.9 µM; MDA-MB-231: 3.2 µM); apoptosis (PANC-1 cancer cells: 28.54% at 17.3 µM | |
| Tersaphilone A-C (84–86) | P. tersa FS441 [42] |
Cytotoxic (Not detected) |
| Tersaphilone D (87) | Cytotoxic (SF-268: 7.5 µM; MCF-7: 7.8 µM; HepG-2: 14.0 µM; A549: 8.3 µM) | |
| Tersaphilone E (88) | Cytotoxic (SF-268: 5.6 µM; MCF-7: 5.4 µM; HepG-2: 9.8 µM; A549: 6.7 µM) | |
| Pleosporales | ||
| Dipleosporalone A (89) | Pleosporales sp. CF09-1 [43] |
Cytotoxic (MDA-MB-231: 1.9 µM; HeLa: 2.5 µM; MGC-803: 1.3 µM; MCF-7: 2.1 µM; A549: 1.0 µM) |
| Dipleosporalone B (90) | Cytotoxic (MDA-MB-231: 3.8 µM; HeLa: 3.0 µM; MGC-803: 2.0 µM; MCF-7: >10 µM; A549: 3.5 µM) | |
| Talaromyces | ||
| Trans-PP-O (91) Atrosins S (92), D (93), E (94), H (95), L (96), M (97), Q (98) and T (99) |
T. atroroseus [17] | Not tested |
| Talaralbol A (100) | T. albobiverticillius [44] | Anti-inflammatory (LPS-induced NO production in RAW264.7 cell: 10.0 µM); 31.0% of inhibitory rate) |
| Talaralbol B (101) | Not detected |
* isolated as endophytic of Polygonatum sibiricum; ** closest relative being Muyocopron atromaculans (MUCL 34983); SF-268 (human glioblastoma carcinoma), MCF-7 (breast cancer), HepG-2 (liver cancer), HeLa (human cervix carcinoma), and A549 (lung cancer), BST = Brine Shrimp test.
2.3. Recent Insights in the Biosynthesis of Azaphilones
The biosynthesis of azaphilones has been reviewed by Pavesi et al. [51] and was also considered in the two latest reviews [16]. Five biosynthetic pathways were exhaustively discussed, which highlighted the comprehensive study of Monascus and Aspergillus pathways [51]. Furthermore, a thorough study performed about the precise role of ammonium nitrate in the production of Monascus pigments showed that some biosynthetic pathways can present changes due to the regulation and expression of several key genes involved [52]. The expression of the gene mppG (MrPigF), responsible for orange pigments, was significantly downregulated with ammonium nitrate addition, and an improvement in yellow pigment production was followed by an upregulated mppE expression. Additionally, ammonium nitrate increased the NH3 content in the fermentation broth resulting in the increased red pigments yield [52].
Dimeric azaphilones have been described in the Chaetonium genus, and the fungal laccase-like multi-copper oxidase gene encoded by CcdJ (CHGG_10025) is believed to dimerize the cochliodones [51]. Cochliodone J (28), a new dimeric azaphilone containing a spirotetrahydropyran moiety, was reported, but the mechanism of the spiro ring formation still remains to be determined [26]. Moreover, the unusual fusion between an eight-membered lactam and a six-membered lactone, presented in the structure of chaetolactama A (38), has not been investigated yet.
The biosynthetic gene cluster responsible for the sequential and convergent production of azaphilones in Chaetonium sp. might count with a hidden gene allegedly responsible for the epimerization of the 7-OH group in chaetoviridin E as well as the oxidation/epoxidation leading to OH groups in C-8a and C-1 positions, followed by methylation of the latter, as in (41) [31]. Based on studies with Monascus, Aspergillus, andTalaromyces, two biosynthetic gene clusters were postulated to drive the diverse azaphilones in H. fragiforme. However, the biosynthetic dimerizations which led to the compounds (42)–(49) demand more investigations. This represents a challenge because Hypoxylaceae azaphilones are exclusively formed during stromata development, which cannot be induced under laboratory conditions [32]. A reasonable proposal consists on a spontaneous aldol condensation responsible for the heterodimerization of different azaphilones derivatives [32].
The biosynthesis of three different azaphilone skeletons was reported for P. tersa FS441. The tersaphilone B (85) showed the unique 6/6-6 carbon skeleton with a cleaved tetrahydrofuranyl ring, and the diastereomers tersaphilones D and E (87–88) displayed a unique five-membered furan ring open and an epoxide ring in C-8a and C-1 positions [42]. A remarkably biosynthetic proposal was provided to penctrimertone (80), which presented a 6/6/6/6 tetracyclic ring system with an unusual aldehyde group in one of the rings [40]. It is supposed to be a citrinin dimer furnished by a citrinin monomer that suffered hydration, oxidation, and reduction affording an orthoquinone methide susceptible to an unusual intermolecular hetero-Diels-Alder reaction with another citrinin molecule [43].
This entry is adapted from the peer-reviewed paper 10.3390/jof7070541
References
- Charlin, V.; Cifuentes, A. A general framework to study the price-color relationship in paintings with an application to Mark Rothko rectangular series. Color Res. Appl. 2021, 46, 168–182.
- Labrecque, L.I. Color research in marketing: Theoretical and technical considerations for conducting rigorous and impactful color research. Psychol. Mark. 2020, 37, 855–863.
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280.
- Meruvu, H.; dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 1–25.
- De Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182.
- Ravi, N.; Keshavayya, J.; Mallikarjuna, M.; Kumar, V.; Zahara, F.N. Synthesis, spectral characterization, anticancer and cyclic voltammetric studies of azo colorants containing thiazole structure. Chem. Data Collect. 2021, 33, 100686.
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271.
- Al Reza, M.S.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F.; et al. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677.
- Bakthavachalu, P.; Kannan, S.M.; Qoronfleh, M.W. Food Color and Autism: A Meta-Analysis. In Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management; Essa, M.M., Qoronfleh, M.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; p. 700.
- Su, C.H.; Tsai, C.H.; Chen, M.H.; Lv, W.Q. U.S. sustainable food market generation Z consumer segments. Sustainability 2019, 11, 3607.
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020, 43, 107601.
- Arikan, E.B.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using Aspergillus carbonarius. J. Fungi 2020, 6, 240.
- Lebeau, J.; Petit, T.; Fouillaud, M.; Dufossé, L.; Caro, Y. Alternative extraction and characterization of nitrogen-containing azaphilone red pigments and ergosterol derivatives from the marine-derived fungal Talaromyces sp. 30570 strain with industrial relevance. Microorganisms 2020, 8, 1920.
- Choe, D.; Song, S.M.; Shin, C.S.; Johnston, T.V.; Ahn, H.J.; Kim, D.; Ku, S. Production and characterization of anti-inflammatory Monascus pigment derivatives. Foods 2020, 9, 858.
- Dufossé, L. Red colourants from filamentous fungi: Are they ready for the food industry? J. Food Compos. Anal. 2018, 69, 156–161.
- Yang, S.-Z.; Huang, Z.-F.; Liu, H.Q.; Hu, X.; Wu, Z.Q. Improving mycelial morphology and adherent growth as well as metabolism of Monascus yellow pigments using nitrate resources. Appl. Microbiol. Biotechnol. 2020, 104, 9607–9617.
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and Biology. Chem. Rev. 2013, 113, 4755–4811.
- Chen, C.; Tao, H.; Chen, W.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Recent advances in the chemistry and biology of azaphilones. RSC Adv. 2020, 10, 10197–10220.
- Isbrandt, T.; Tolborg, G.; Ødum, A.; Workman, M.; Larsen, T.O. Atrorosins: A new subgroup of Monascus pigments from Talaromyces atroroseus. Appl. Microbiol. Biotechnol. 2020, 104, 615–622.
- Isbrandt, T.; Frisvad, J.C.; Madsen, A.; Larsen, T.O. New azaphilones from Aspergillus neoglaber. AMB Express 2020, 10, 145.
- Petersen, T.I.; Kroll-Møller, P.; Larsen, T.O.; Ødum, A.S.R. A Novel Class of Pigments in. Aspergillus. Patent No. WO2020094830, 8 November 2019.
- El-Kashef, D.H.; Youssef, F.S.; Hartmann, R.; Knedel, T.-O.; Janiak, C.; Lin, W.; Reimche, I.; Teusch, N.; Liu, Z.; Proksch, P. Azaphilones from the red sea fungus Aspergillus falconensis. Mar. Drugs 2020, 18, 204.
- Gu, L.; Sun, F.-J.; Li, C.-P.; Cui, L.-T.; Yang, M.-H.; Kong, L.-Y. Ardeemins and citrinin dimer derivatives from Aspergillus terreus harbored in Pinellia ternate. Phytochem. Lett. 2021, 42, 77–81.
- Gao, W.; Chai, C.; Li, X.-N.; Sun, W.; Li, F.; Chen, C.; Wang, J.; Zhu, H.; Wang, Y.; Hu, Z.; et al. Two anti-inflammatory chlorinated azaphilones from Chaetomium globosum TW1-1 cultured with 1-methyl-L-tryptophan and structure revision of chaephilone C. Tetrahedron Lett. 2020, 61, 151516.
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 2018, 16, 61.
- Song, C.; Ding, G.; Wu, G.; Yang, J.; Zhang, M.; Wang, H.; Wei, D.; Qin, J.; Guo, L. Identification of a Unique Azaphilone Produced by Chaetomium globosum Isolated from Polygonatum sibiricum. Chem. Biodivers. 2020, 17, e1900744.
- Sarmales-Murga, C.; Akaoka, F.; Sato, M.; Takanishi, J.; Mino, T.; Miyoshi, N.; Watanabe, K. A new class of dimeric product isolated from the fungus Chaetomium globosum: Evaluation of chemical structure and biological activity. J. Antibiot. 2020, 73, 320–323.
- Wang, W.; Yang, J.; Liao, Y.-Y.; Cheng, G.; Chen, J.; Cheng, X.-D.; Qin, J.J.; Shao, Z. Cytotoxic Nitrogenated Azaphilones from the Deep-Sea-Derived Fungus Chaetomium globosum MP4-S01-7. J. Nat. Prod. 2020, 83, 1157–1166.
- Wani, N.; Khanday, W.; Tirumale, S. Evaluation of anticancer activity of Chaetomium cupreum extracts against human breast adenocarcinoma cell lines. Matrix Sci. Pharma 2020, 4, 31.
- Yamada, T.; Muroga, Y.; Tanaka, R. New azaphilones, seco-chaetomugilins A and D, produced by a marine-fish-derived Chaetomium globosum. Mar. Drugs 2009, 7, 249–257.
- Zu, W.-Y.; Tang, J.-W.; Hu, K.; Zhou, Y.-F.; Gou, L.-L.; Su, X.-Z.; Lei, X.; Sun, H.-D.; Puno, P.-T. Chaetolactam A, an Azaphilone Derivative from the Endophytic Fungus Chaetomium sp. g1. J. Org. Chem. 2021, 86, 475–483.
- Zhang, X.-Y.; Tan, X.-M.; Yu, M.; Yang, J.; Sun, B.-D.; Qin, J.-C.; Guo, L.-P.; Ding, G. Bioactive metabolites from the desert plant-associated endophytic fungus Chaetomium globosum (Chaetomiaceae). Phytochemistry 2021, 185, 112701.
- Becker, K.; Pfütze, S.; Kuhnert, E.; Cox, R.J.; Stadler, M.; Surup, F. Hybridorubrins A–D: Azaphilone Heterodimers from Stromata of Hypoxylon fragiforme and Insights into the Biosynthetic Machinery for Azaphilone Diversification. Chem. A Eur. J. 2021, 27, 1438–1450.
- Surup, F.; Narmani, A.; Wendt, L.; Pfütze, S.; Kretz, R.; Becker, K.; Menbrivès, C.; Giosa, A.; Elliott, M.; Petit, C.; et al. Identification of fungal fossils and novel azaphilone pigments in ancient carbonised specimens of Hypoxylon fragiforme from forest soils of Châtillon-sur-Seine (Burgundy). Fungal Divers. 2018, 92, 345–356.
- Lambert, C.; Pourmoghaddam, M.J.; Cedeño-Sanchez, M.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stradal, T.E.B.; Stadler, M. Resolution of the Hypoxylon fuscum complex (hypoxylaceae, xylariales) and discovery and biological characterization of two of its prominent secondary metabolites. J. Fungi 2021, 7, 131.
- Wu, H.-C.; Chen, J.-J.; Wu, M.-D.; Cheng, M.-J.; Chang, H.-S. Identification of new pigments produced by the fermented rice of the fungus Monascus pilosus and their anti-inflammatory activity. Phytochem. Lett. 2020, 40, 181–187.
- Nakashima, K.I.; Tomida, J.; Tsuboi, T.; Kawamura, Y.; Inoue, M. Muyocopronones A and B: Azaphilones from the endophytic fungus Muyocopron laterale. Beilstein J. Org. Chem. 2020, 16, 2100–2107.
- Cain, J.W.; Miller, K.I.; Kalaitzis, J.A.; Chau, R.; Neilan, B.A. Genome mining of a fungal endophyte of Taxus yunnanensis (Chinese yew) leads to the discovery of a novel azaphilone polyketide, lijiquinone. Microb. Biotechnol. 2020, 13, 1415–1427.
- Sabdaningsih, A.; Liu, Y.; Mettal, U.; Heep, J.; Wang, L.; Cristianawati, O.; Nuryadi, H.; Sibero, M.T.; Marner, M.; Radjasa, O.K.; et al. A new citrinin derivative from the Indonesian marine sponge-associated fungus Penicillium citrinum. Mar. Drugs 2020, 18, 227.
- Wei, Q.; Bai, J.; Yan, D.; Bao, X.; Li, W.; Liu, B.; Zhang, D.; Qi, X.; Yu, D.; Hu, Y. Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 2021, 11, 572–587.
- Li, H.-T.; Duan, R.-T.; Liu, T.; Yang, R.-N.; Wang, J.-P.; Liu, S.-X.; Yang, Y.B.; Zhou, H.; Ding, Z.-T. Penctrimertone, a bioactive citrinin dimer from the endophytic fungus Penicillium sp. T2-11. Fitoterapia 2020, 146, 104711.
- Yang, Z.-J.; Zhang, Y.-F.; Wu, K.; Xu, Y.-X.; Meng, X.-G.; Jiang, Z.-T.; Ge, M.; Shao, L. New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No.5416. Fitoterapia 2020, 145, 104573.
- Chen, S.; Liu, Z.; Chen, Y.; Tan, H.; Liu, H.; Zhang, W. Tersaphilones A-E, cytotoxic chlorinated azaphilones from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron 2021, 78, 131806.
- Cao, F.; Meng, Z.-H.; Wang, P.; Luo, D.-Q.; Zhu, H.-J. Dipleosporalones A and B, Dimeric Azaphilones from a Marine-Derived Pleosporales sp. Fungus. J. Nat. Prod. 2020, 83, 1283–1287.
- Bai, W.; Jing, L.-L.; Guan, Q.-Y.; Tan, R.-X. Two new azaphilone pigments from Talaromyces albobiverticillius and their anti-inflammatory activity. J. Asian Nat. Prod. Res. 2021, 23, 325–332.
- Choe, D.; Jang, H.; Jung, H.H.; Shin, C.S.; Johnston, T.V.; Kim, D.; Ku, S. In vivo anti-obesity effects of Monascus pigment threonine derivative with enhanced hydrophilicity. J. Funct. Foods 2020, 67, 103849.
- Skariyachan, S.; Pius, S.; Gopal, D.; Muddebihalkar, A.G. Natural lead molecules probably act as potential inhibitors against prospective targets of SARS-CoV-2: Therapeutic insight for COVID-19 from computational modelling, molecular docking and dynamic simulation studies. Chemistry 2020. preprint.
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866.
- Zhang, S.-P.; Huang, R.; Li, F.-F.; Wei, H.-X.; Fang, X.-W.; Xie, X.-S.; Lin, D.-G.; Wu, S.-H.; He, J. Antiviral anthraquinones and azaphilones produced by an endophytic fungus Nigrospora sp. from Aconitum carmichaeli. Fitoterapia 2016, 112, 85–89.
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52.
- Kim, J.-Y.; Woo, E.-E.; Ha, L.S.; Ki, D.-W.; Lee, I.-K.; Yun, B.-S. Neuraminidase Inhibitors from the Fruiting Body of Glaziella splendens. Mycobiology 2019, 47, 256–260.
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071.
- Chen, D.; Wang, Y.; Chen, M.; Fan, P.; Li, G.; Wang, C. Ammonium nitrate regulated the color characteristic changes of pigments in Monascus purpureus M9. AMB Express 2021, 11, 3.
This entry is offline, you can click here to edit this entry!